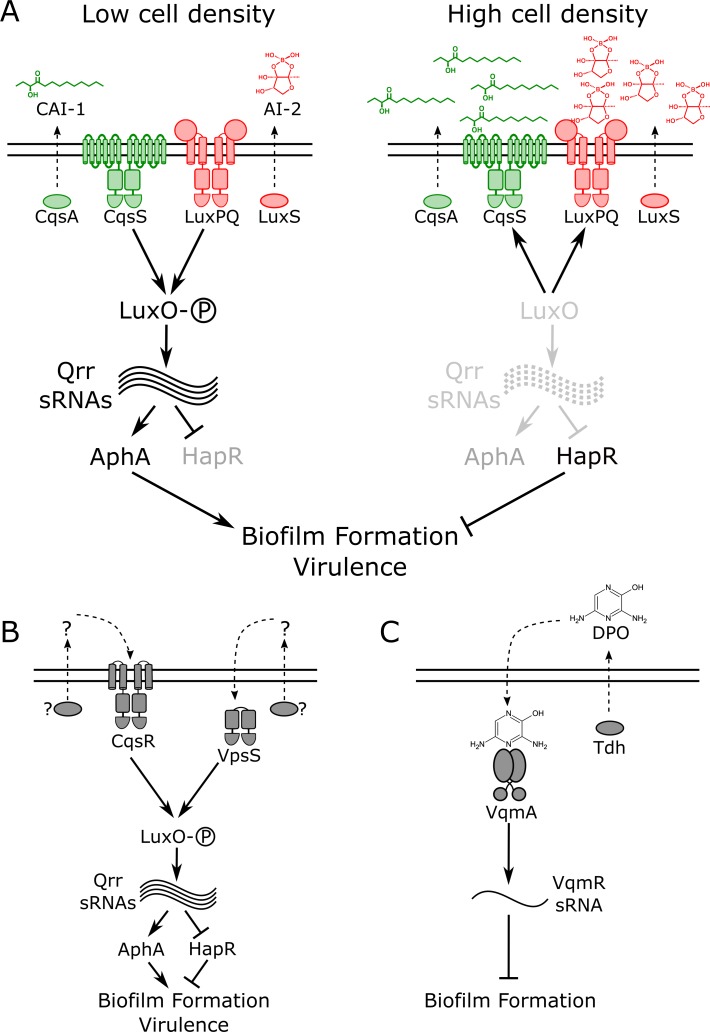
Fig 1. Simplified V. cholerae QS circuits.
(A) Two established autoinducer–receptor pairs control QS behaviors in V. cholerae. One autoinducer–receptor pair consists of CAI-1, which is synthesized by CqsA and detected by the two-component sensor-histidine kinase, CqsS. The second autoinducer–receptor pair is AI-2, produced by LuxS and detected by LuxPQ, also a two-component sensor-histidine kinase. At LCD (left), both receptors act as kinases that promote phosphorylation of the response regulator, LuxO. Phosphorylated LuxO activates expression of genes encoding regulatory RNAs called the Qrr sRNAs. The Qrr sRNAs activate production of the LCD master regulator, AphA, and repress production of the HCD master regulator, HapR. These conditions drive biofilm formation and virulence factor production. At HCD (right), the autoinducer-bound receptors act as phosphatases that strip phosphate from LuxO, resulting in AphA repression and HapR production, conditions that promote the free-swimming, planktonic lifestyle and repression of virulence. (B) Two additional QS receptors, VpsS and CqsR, also funnel information into LuxO. Their cognate autoinducers and autoinducer synthases are not known. (C) A recently discovered QS pathway consists of the autoinducer DPO, synthesized by Tdh, and its partner receptor VqmA. At HCD, DPO-bound VqmA activates expression of a gene encoding a sRNA called VqmR. VqmR represses biofilm formation. AI-2, autoinducer-2; CAI-1, cholerae autoinducer-1; DPO, 3,5-dimethylpyrazin-2-ol; HCD, high cell density; LCD, low cell density; QS, quorum sensing; sRNA, small regulatory RNA; Tdh, threonine dehydrogenase.