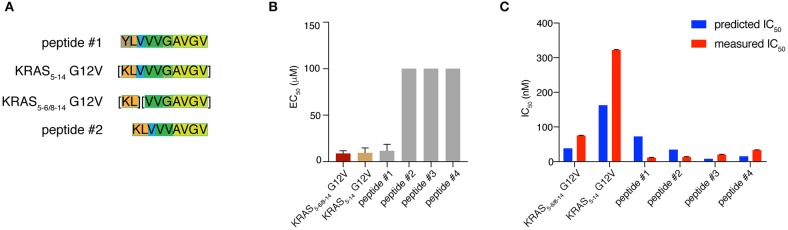
Figure 6.
KRAS5−6/8−14 G12V epitope candidate is efficiently transported by TAPs and strongly binds HLA-A*02:01 complex. (A) Sequence comparison between KRAS5−6/8−14 G12V [KL][VVGAVGV] and KRAS5−14 G12V [KLVVVGAVGV] epitope candidates as well as their modified versions (peptides #1 YLVVVGAVGV and #2 KLVVVAVGV). Common sequences among peptides are color-coded. (B) Transport efficiency into the ER lumen mediated by TAPs of KRAS5−6/8−14 G12V and KRAS5−14 G12V epitope candidates, their modified versions (peptides #1 and #2) and two unrelated peptides (peptides #3 and #4; Supplementary Table 2). The EC50 was computed using a competing peptide as reference. We here report the EC50 values obtained upon subtracting the peptide transport in absence of ATP. (C) Predicted and measured binding affinities of the peptides to the HLA-A*02:01 complex. Binding affinity prediction was carried out with the NetMHCPan 3.0 algorithm. In (B,C) mean and SD of biological replicates (bars) are shown.