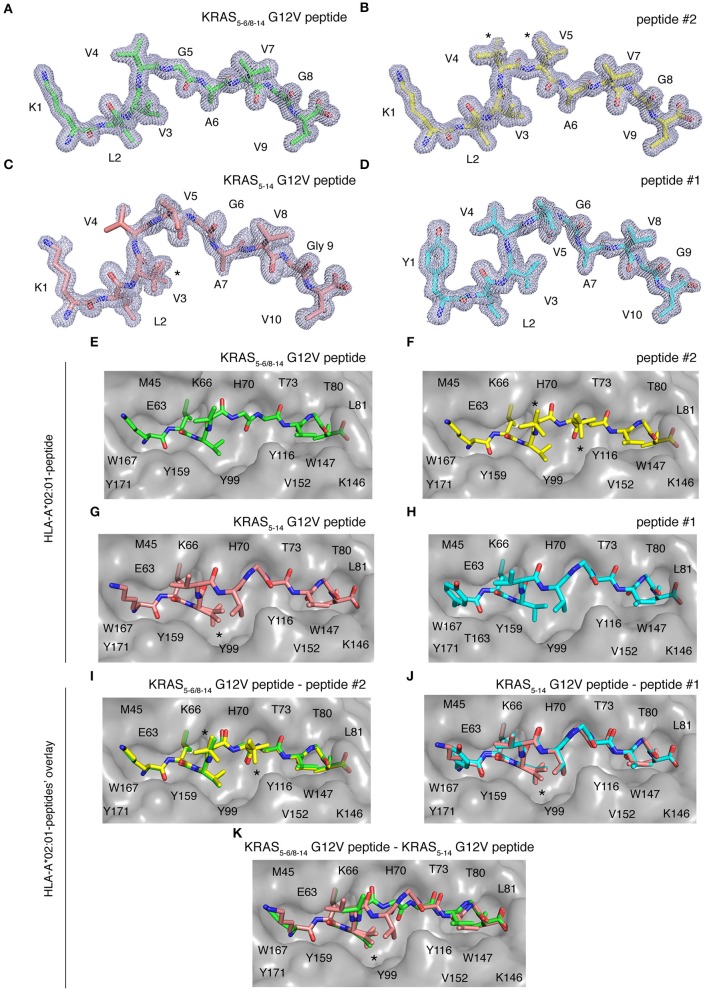
Figure 7.
HLA-A*02:01-KRAS G12V peptides binding mode. Binding mode of spliced epitope candidate KRAS5−6/8−14 G12V [KL][VVGAVGV], non-spliced epitope candidate KRAS5−14 G12V [KLVVVGAVGV], peptides #1 YLVVVGAVGV and #2 KLVVVAVGV to HLA-A*02:01 complex. (A–D) 2Fo-Fc electron density map contoured at 1σ for KRAS5−6/8−14 G12V peptide (A), peptide #2 (B), non-spliced peptide KRAS5−14 G12V (C), and peptide #1 (D). (E–H) Binding of spliced peptide KRAS5−6/8−14 G12V (E; green sticks), peptide #2 (F; yellow sticks), non-spliced peptide KRAS5−14 G12V (G; brown sticks), and peptide #1 (H; cyan sticks) to HLA-A*02:01 protein (gray molecular surface). (I–K) Overlay of KRAS5−6/8−14 G12V and peptide #2 binding to HLA-A*02:01 (I), KRAS5−14 G12V peptide and peptide #1 (J), as well as KRAS5−6/8−14 G12V and KRAS5−14 G12V peptides (K) binding to HLA-A*02:01 molecule. All peptides are shown as sticks and in (A–D), peptide residues are labeled with one-letter amino acid codes. In (E–K), the residues of HLA-A*02:01 that are extended in the peptide binding interface are labeled with single-letter amino acid codes. In (B,F,I), * indicates the alternate conformations for residues V4 and V5 of peptide #2. In (C,G,J–K), *indicates the alternate conformations for residues V3 of KRAS5−14 G12V peptide.