Abstract
Background
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone that is increased in azotemic cats with chronic kidney disease (CKD) and predictive of the onset of azotemia in older cats. The introduction of symmetric dimethylarginine (SDMA) as a biomarker of glomerular filtration rate has led to the identification of cats in which SDMA is increased, but plasma creatinine concentrations remains within reference range. There is currently little understanding of the metabolic changes present in such cats.
Objectives
To examine the relationship between plasma FGF23 and SDMA concentrations in non‐azotemic geriatric cats.
Animals
Records of a cross section of client‐owned cats (n = 143) without azotemic CKD.
Methods
Clinicopathological information was obtained from cats (≥ 9 years) from records of 2 first opinion practices. The relationship between plasma SDMA and FGF23 concentrations was examined using Spearman's correlation and variables compared using the Mann‐Whitney U test.
Results
Cats with increased SDMA concentrations had significantly higher plasma FGF23 (P < .001) and creatinine (P < .001) concentrations compared to cats with SDMA concentrations within reference range. A weak positive relationship was demonstrated between plasma FGF23 and SDMA concentrations (r = .35, P < .001) and between plasma FGF23 and creatinine (r = .23, P = .005) concentrations.
Conclusions and Clinical Importance
More cats with increased SDMA concentrations had higher FGF23 concentrations than those with SDMA concentrations within the reference range, suggesting the presence of an alteration in phosphate homeostasis. Further studies are warranted to identify influencing factors and to explore the utility of FGF23 concentration to inform management of cats with early stage CKD.
Keywords: azotemia, feline, phosphate, renal
Abbreviations
- 1,25 vitamin D3
25‐dihydroxyvitamin D
- AKI
acute kidney injury
- CKD
chronic kidney disease
- CKD‐MBD
chronic kidney disease mineral and bone disorder
- EDTA
ethylenediaminetetraacetic acid
- eGFR
estimated GFR
- ELISA
enzyme‐linked immunosorbent assay
- FGF23
fibroblast growth factor 23
- FGFR
fibroblast growth factor receptor
- GFR
glomerular filtration rate
- iFGF23
intact FGF23
- IRIS
International Renal Interest Society
- PCV
packed cell volume
- PTH
parathyroid hormone
- SDMA
symmetric dimethylarginine
- tCa
total calcium
- tMg
total magnesium
- TT4
total thyroxine
1. INTRODUCTION
Symmetric dimethylarginine (SDMA), a methylarginine produced in the nucleus of all cells,1 is cleared primarily by the kidneys. Plasma SDMA concentration has been shown to have a strong negative correlation with glomerular filtration rate (GFR) and a strong positive correlation with plasma creatinine concentration in both humans and cats,1, 2, 3 highlighting its suitability as a renal biomarker. Importantly, in a population of geriatric cats with naturally occurring chronic kidney disease (CKD), plasma SDMA concentration has been shown to increase an average of 17 months before an increase in plasma creatinine concentration above its upper reference range.4 In recent years, International Research Interest Society (IRIS) guidelines5 have been modified to include a persistent increase in SDMA concentration above reference range, with a plasma creatinine concentration of <1.6 mg/dL (140 μmol/L) in cats as a criterion for classification of IRIS Stage 1 CKD and indicative of decreased renal function.
Fibroblast growth factor 23 (FGF23) is a key regulatory hormone in phosphate homeostasis. Consisting of 251 amino acids, it is secreted by osteoblasts and osteocytes and binds to fibroblast growth factor receptors (FGFR), specifically FGFR1c, which is widely expressed throughout the body.6 Within renal tubular epithelial cells, FGF23‐Klotho‐FGFR1c initiates mitogen‐activated protein kinase, extracellular signal‐related kinase‐1/2, or early growth response‐1 pathways.7 These signals act to downregulate expression of NaPi2a cotransporters resulting in decreased reabsorption of phosphate and increased phosphaturia.
In CKD, a decrease in GFR and subsequent increase in serum phosphate concentration cause a disruption in phosphate‐calcium homeostasis.8 Chronic kidney disease‐mineral and bone disorder (CKD‐MBD) describes a syndrome observed in humans with CKD involving derangements in blood concentrations of FGF23, parathyroid hormone (PTH),1, 25‐dihydroxyvitamin D3 (1,25 vitamin D3, calcitriol), calcium and phosphate, with accompanying renal osteodystrophy and vascular and soft tissue calcification.9 In humans with CKD‐MBD, it is well established that an increase in serum FGF23 concentration precedes an increase in serum phosphate concentration.10 Furthermore, in all stages, including early stage CKD, the prevalence of FGF23 excess has been shown to be higher than that of other hormonal derangements, suggesting that identification of FGF23 excess may be the earliest indicator of an adaptive mechanism for deranged phosphate homeostasis in CKD.10
In cats with CKD, the same mineral and hormone derangements occur. Fibroblast growth factor 23 and PTH have been shown to be increased despite normal plasma total calcium (tCa) and phosphorus concentrations in cats that develop azotemic CKD compared to those that remain non‐azotemic, suggesting the presence of altered phosphate homeostasis11, 12 in early stage non‐azotemic CKD. No published studies have examined the metabolic status of non‐azotemic cats with increased SDMA, which may be considered to have early stage CKD.
Our aim was to examine the relationship between plasma FGF23 and SDMA concentrations in geriatric non‐azotemic cats.
2. METHODS
2.1. Case selection
Records from a geriatric cat clinic held at 2 London‐based first opinion practices (Beaumont Sainsbury Animal Hospital in Camden and People's Dispensary for Sick Animals in Bow) between October 2011 and December 2016 were reviewed and cases were retrospectively selected. The clinic prospectively recruits apparently healthy cats >9 years of age for wellness screening and longitudinal monitoring every 6 months, specifically to evaluate for the development of systemic hypertension, CKD hyperthyroidism, or some combination of these.
Records of cats that were azotemic or had a history of azotemia, plasma total thyroxine (TT4) concentration > 40 nmol/L (reference range, 10‐55 nmol/L), received medical treatment for hyperthyroidism, or had diabetes mellitus were excluded from the analysis. Cats receiving treatment for systemic hypertension with amlodipine besylate were included. Cats were included in the analysis if they had plasma FGF23 or SDMA concentration recorded and sufficient stored sample to measure required variables that had not been quantified previously. In cats that had been evaluated at the clinic multiple times during the study period, only the first recorded visit was included in the analysis.
The following historical, physical examination findings and biochemical data were required and extracted from the records: plasma creatinine, urea, phosphate, tCa, total magnesium (tMg), TT4, SDMA, FGF23 and PTH concentrations, packed cell volume (PCV), urine specific gravity, age, weight, body condition score, muscle condition score,13 breed, and sex.
2.2. Blood and urine sampling
All cats initially were examined and samples collected and stored under the approval of the Ethics and Welfare Committee of the Royal Veterinary College and with informed consent of the owner. Blood samples had been obtained by jugular venipuncture and transferred to ethylenediaminetetraacetic acid (EDTA) and heparinized tubes. Urine samples were collected by cystocentesis. According to clinic protocol for sample processing, samples were stored at 4°C after collection, for a maximum of 6 hours before centrifugation and separation. Heparinized plasma was submitted to an external laboratory (IDEXX laboratories, Wetherby, United Kingdom) for biochemical analysis on the day of sample collection. Residual heparinized and EDTA plasma samples were stored at −80°C for future batch analysis. Where measurement had not already been performed, EDTA plasma samples were used to measure PTH concentrations using a total intact PTH immunoradiometric assay previously validated for cats (Total intact PTH immunoradiometric assay‐coated bead version, 3KG600, Scantibodies, Santee, California).14 The lower limit of detection of the assay was determined to be 5.2 pg/mL. Therefore, any samples with PTH concentration <5.2 pg/mL were arbitrarily assigned a value of 2.6 pg/mL. Intact plasma FGF23 concentration was measured using EDTA plasma samples using a previously validated enzyme‐linked immunosorbent assay (ELISA; FGF23 ELISA Kit, Kainos Laboratories, Tokyo, Japan).15 Stored heparin plasma samples were sent to an external laboratory for SDMA quantification. Urine specific gravity was determined by refractometry on urine samples on the day of collection.
2.3. Statistical analysis
Statistical analyses were performed using commercial software (IBM SPSS Statistics for Windows, Version 24, IBM, Corp, Armonk, New York; GraphPad Prism 7, GraphPad Software, La Jolla, California). Statistical significance was set at P < .05. Normality of the numerical data distribution was assessed by visual inspection of histograms. The majority of data was not normally distributed and for consistency all numerical clinical data is presented as median (25th and 75th percentiles). Cats were grouped into those with SDMA concentration above reference range (>14 μg/dL) and those with SDMA within reference interval (≤14 μg/dL).16 Comparisons were made between groups using Mann‐Whitney U tests and relationships between numerical variables were evaluated using Spearman's correlation.
3. RESULTS
Between October 2011 and December 2016, 876 non‐azotaemic cats were identified, of which 422 were excluded because of suspected or documented hyperthyroidism and 11 because of documented historical azotemia. Of the remaining 443 cats, 300 did not have recorded either a plasma FGF23 or SDMA concentration or had insufficient stored EDTA or heparin plasma sample available for measurement of either plasma FGF23 or SDMA.
The study population consisted of 143 cats; 76 females (3 intact) and 67 males (1 intact). Most common breeds were domestic short hair (n = 109) and domestic long hair (n = 23), Birman (n = 2), Siamese (n = 2), British Short Hair (n = 2), and 1 each of Birman cross, Devon Rex, Persian, Norwegian Forest, and Burmese. Eighty cats had plasma SDMA concentration measured at the time of sampling; the remaining 63 cats had SDMA measured on stored heparin plasma within 70 months of sample collection. All 143 cats had plasma FGF23 and PTH concentrations measured on stored EDTA plasma within 18 months of sample date. Of the 143 cats, 107 had plasma SDMA concentrations within the reference range (≤14 μg/dL) and 36 had increased plasma SDMA concentrations. Clinicopathological variables for each group are found in Table 1.
Table 1.
Clinicopathological characteristics of cats grouped according to SDMA status
| SDMA within RI (n = 107) | n | SDMA above RI (n = 36) | n | P value | |
|---|---|---|---|---|---|
| SDMA (1‐14 μg/dL) | 10 [10, 11] | 107 | 15 [15, 19] | 36 | |
| Age (years) | 12.2 [11.2, 14.5] | 107 | 14 [12.1, 15.3] | 36 | .66 |
| Weight (kg) | 4.35 [3.36, 5.08] | 107 | 4.01 [3.02, 4.85] | 36 | .22 |
| BCS (1‐9) | 5 [4, 6] | 107 | 5 [3, 5] | 36 | .19 |
| MCS (0‐2) | 1 [1, 2] | 105 | 1 [1, 1] | 36 | .06 |
| Sex (male n [%]) | 40 | 107 | 54 | 36 | .25 |
| PCV (%) | 38 [25, 40] | 106 | 35 [33, 40] | 36 | .12 |
| Creatinine (0.23‐2.00 mg/dL) | 1.49 [1.31, 1.67] | 107 | 1.76 [1.59, 1.89] | 36 | <.001 |
| FGF23 (56‐700) pg/mL) | 155 [127, 243] | 107 | 271 [176, 523] | 36 | <.001 |
| PTH (2.6‐17.6 pg/mL) | 7 [2.6, 11.3] | 44 | 7.3 [4.1, 9.3] | 10 | .84 |
| Phosphate (2.71‐6.89 mg/dL) | 3.66 [3.22, 4.12] | 107 | 3.84 [3.28, 4.18] | 36 | .40 |
| Total calcium (8.22‐11.82 mg/dL) | 9.7 [9.3, 10.1] | 107 | 9.98 [9.5, 10.18] | 36 | .70 |
| Total magnesium (1.73‐2.57 mg/dL) | 2.14 [2.04, 2.26] | 36 | 1.97 [1.87, 2.21] | 5 | .62 |
Note: Data presented as median [25th, 75th percentile] or prevalence (n [%]).
Abbreviations: BCS, body condition score; FGF23, fibroblast growth factor 23; MCS, muscle condition score; PCV, packed cell volume; PTH, parathyroid hormone; RI, reference interval; SDMA, symmetric dimethylarginine.
Outliers were identified: 1 cat with plasma SDMA concentration of 63 μg/dL and 3 cats with plasma FGF23 concentrations of 1345, 1502, and 1300 pg/mL. Analysis was repeated without outliers; results did not differ. All stated P values are for the whole cohort, including outliers.
A significant weak positive correlation was found between plasma FGF23 and SDMA concentrations (r = .353; P < .001; Figure 1). A significant moderate positive correlation was found between plasma creatinine and SDMA concentrations (r = .525; P < .001; Figure 2). A significant weak positive correlation was found between plasma FGF23 and creatinine concentrations (r = .232, P = .005; Figure 3). No significant correlation was found between plasma FGF23 and phosphate concentrations (P = .22) or plasma FGF23 and tMg concentrations (P = .60).
Figure 1.
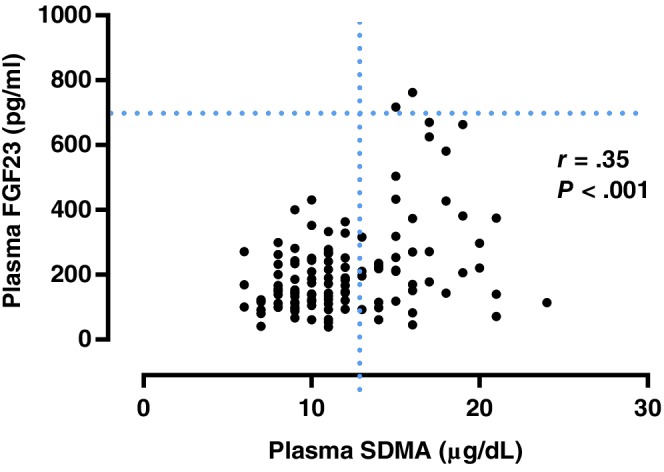
Scatter plot illustrating the relationship between plasma symmetric dimethylarginine (SDMA) and plasma fibroblast growth factor 23 (FGF23) concentration. The broken lines represent upper reference interval for plasma SDMA16 (14 μg/dL) and plasma FGF2315 (700 pg/mL) concentrations. Outliers have been omitted from the graph
Figure 2.
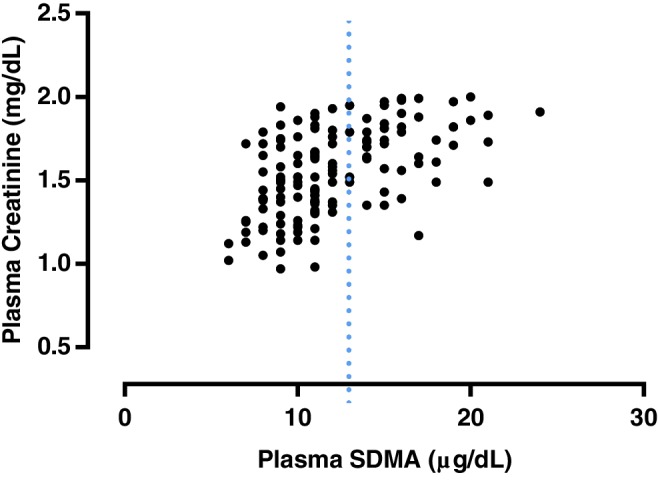
Scatter plot illustrating the relationship between plasma symmetric dimethylarginine (SDMA) and plasma creatinine concentration. The broken line represents the upper reference interval for plasma SDMA16 concentration (14 μg/dL). Outliers have been omitted from the graph
Figure 3.
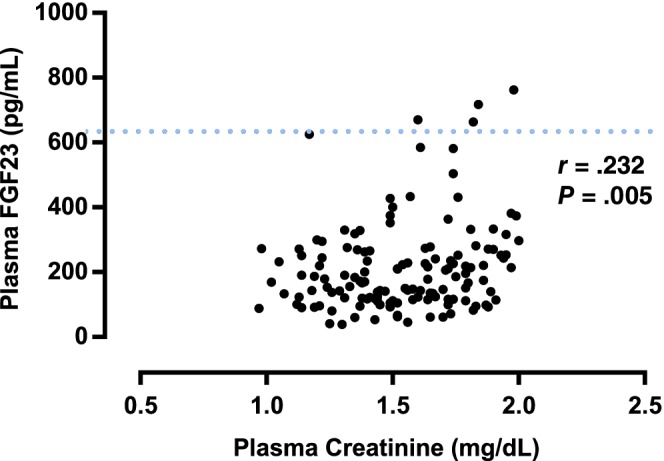
Scatter plot illustrating the relationship between plasma creatinine and plasma fibroblast growth factor 23 (FGF23) concentration. The broken line represents the upper reference interval for plasma FGF23 concentration15 (700 pg/mL). Outliers have been omitted from the graph
Plasma FGF23 concentrations and plasma creatinine concentrations were significantly higher in cats with plasma SDMA concentrations above the reference range (>14 μg/dL; P < .001; Figure 4). No other significant differences in clinicopathological variables between SDMA groups were observed (Table 1 and Figure 4).
Figure 4.
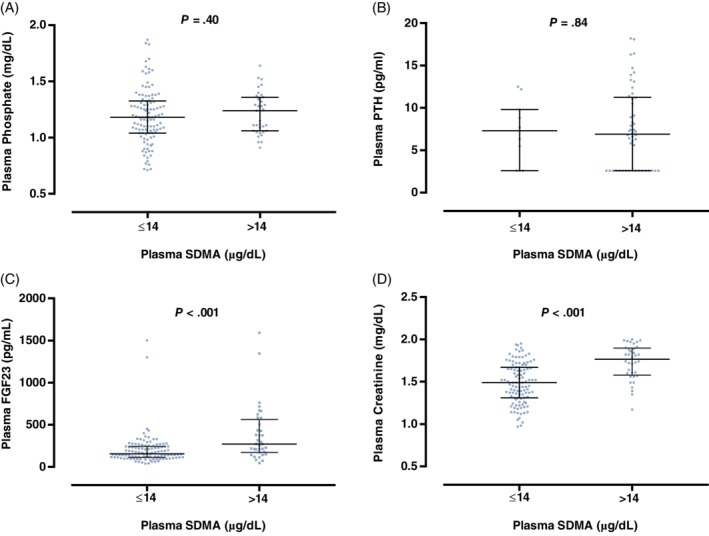
Box plot illustrating A, plasma phosphate; B, parathyroid hormone (PTH); C, fibroblast growth factor 23 (FGF23); and D, creatinine concentration for cats grouped according to symmetric dimethylarginine (SDMA) status: SDMA within reference interval (≤14 μg/dL); SDMA above upper reference interval (>14 μg/dL).16 The boxes represent the 25th and 75th percentiles and the central lines represent median values. C, The broken line represents the upper reference interval for plasma FGF2315 concentration (700 pg/mL)
4. DISCUSSION
Results of our retrospective study show that more cats with plasma SDMA concentration above the reference range had significantly higher plasma FGF23 and creatinine concentrations than did cats with plasma SDMA concentration within reference interval, with no significant difference in plasma phosphate, tCa or PTH concentrations between groups. We also identified a positive correlation between plasma FGF23 and both plasma SDMA and creatinine concentrations and a strong positive correlation between plasma SDMA and plasma creatinine concentration. The strength of these correlations is likely to have been influenced by the limited range of stages of CKD included in the study, because the intent was to evaluate early IRIS stage CKD.
Plasma SDMA and creatinine concentrations are established indirect biomarkers of GFR,2, 17 and the positive correlation observed in our study reflects previous studies. The retrospective nature of our study meant that GFR measurement was not performed, but the significantly higher plasma creatinine concentration in those cats with increased plasma SDMA concentrations compared to those with SDMA concentrations within the reference range is supportive of an early decrease in GFR in these cats. Although, it should be recognized that a limitation of this study is the single plasma SDMA concentration used to classify cats, IRIS recommendations specify a persistent increase in SDMA on ≥2 occasions to be used in clinical practice.5 The weak positive relationship of plasma FGF23 concentration with both plasma creatinine and SDMA concentrations was expected, given that SDMA and creatinine are established indirect biomarkers of GFR and plasma FGF23 concentration is known to increase with decreasing GFR.18 However, evidence exists that GFR is not the only factor influencing FGF23 concentrations.
In human medicine, it has been well established that plasma FGF23 concentration increases in patients with CKD and that increases in plasma FGF23 and PTH concentrations occur before hyperphosphatemia.10, 18, 19 A cross‐sectional study of nearly 3879 CKD patients10 indicated the prevalence of increased FGF23 concentration was higher than that of increased PTH concentration across all levels of estimated GFR (eGFR), including those patients with the most preserved eGFR, and it has been suggested that increased plasma FGF23 concentration is the earliest indicator of a disordered mineral metabolism in CKD. The most striking result of our study was the significantly higher plasma FGF23 concentration despite no significant difference in plasma phosphate or PTH concentrations in cats with increased plasma SDMA concentrations.
Previous studies of client‐owned cats >9 years of age have shown increases in both plasma FGF23 and PTH concentrations to be predictive of the onset of azotemia.11, 12 Interestingly, in another study,10 the proportion of patients with increased plasma FGF23 concentrations and normal PTH concentrations was significantly higher than the proportion of patients with increased PTH concentrations only across all stages of CKD, reflecting our findings that plasma PTH concentration was not significantly different in those cats with increased plasma SDMA concentration compared to those with plasma SDMA concentration within reference range and considered to have normal renal function. However, it also should be recognized that in our study, plasma PTH concentrations were available in only 38% of cases with increased plasma SDMA concentrations, which may have led to insufficient statistical power to identify a significant difference between groups. Interestingly, a recent meta‐analysis of 611 studies examining the relationship among eGFR, plasma FGF23, and soluble α‐klotho concentrations in patients with CKD observed a significant positive correlation between eGFR and soluble α‐klotho and a significant negative correlation between soluble α‐klotho and FGF23 concentrations.20 Significant negative correlation between plasma soluble α‐klotho and FGF23, phosphate, and PTH concentrations in CKD patients also has been identified.21 Soluble α‐klotho, the functional circulating form of the transmembrane bound coreceptor for FGF23, may be an early biomarker for a decrease in GFR and also a marker for CKD‐MBD.21 The relationship of plasma soluble α‐klotho concentration with both GFR and CKD‐MBD has not yet been studied in cats with CKD.
Multiple mechanisms for an increase in plasma FGF23 concentration in early CKD have been proposed, principally as an adaptive mechanism to disturbed phosphate homeostasis and as a result of decreased renal clearance.19, 22, 23 A strong inverse correlation between GFR and circulating FGF23 concentration has been established in humans18 and reported in cats.12 However, a number of studies have shown increased bone expression of FGF23 in all stages of CKD including early stages and also in acute kidney injury (AKI), supporting the hypothesis that increased plasma FGF23 concentration in early CKD is primarily caused by increased secretion rather than solely a decrease in renal clearance. In a study of 32 human patients with CKD Stages 2 to 5, immunohistochemical analysis of biopsy samples from the iliac crest identified increased FGF23 expression in early CKD which was shown to correlate positively with plasma FGF23 concentration (r = .43, P < .01).24 Interestingly, in 2 animal models of toxin‐induced AKI,25 in which plasma FGF23 concentrations were shown to increase rapidly, immunohistochemical and Western blot analysis of femoral bone indicated that bone concentrations of FGF23 were 2‐fold higher than in controls. Furthermore, in the same model, the half‐life of recombinant human FGF23 administered IV was only modestly increased in mice with AKI compared to controls, suggesting that decreased renal clearance is not the primary mechanism for increased circulating FGF23 in AKI and CKD, although it is recognized that the mechanisms leading to increased plasma FGF23 concentration in AKI may be different than those in CKD.
In cats, plasma FGF23 concentration has been shown to be higher in hyperphosphatemic cats with azotemic CKD when compared with normophosphatemic cats at the same IRIS stage.15 Furthermore, circulating FGF23 concentrations have been shown to decrease in cats with azotemic CKD fed a phosphorous‐restricted diet when compared to controls.15 In addition, increasing phosphorus intake in cats over a period of 28 weeks led to significantly increased plasma FGF23 concentrations without changes in GFR or, initially, plasma PTH concentration and was associated with a decrease in plasma phosphate concentration.26 Collectively these studies suggest that increased secretion rather than decreased renal elimination is likely to be responsible for the increase in plasma FGF23 concentration in early CKD, indicating that an alteration in phosphate homeostasis may be present. In our study, the 25th and 75th percentiles for plasma FGF23 concentration in those cats with increased plasma SDMA concentrations were 176 and 523 pg/mL. The distribution of FGF23 concentrations in this group of cats may indicate that only some cats with early CKD have an alteration in phosphate homeostasis which could support the diagnostic utility of plasma FGF23 concentration in identifying those cats.
The exact physiological stimuli for FGF23 secretion are not yet fully understood. In vivo, FGF23 expression is upregulated by phosphate or 1,25 vitamin D37, 27 and in vitro extracellular phosphate has been shown to stimulate FGF23 expression and a variety of genes associated with phosphate handling or 1,25 vitamin D3 metabolism in IDG‐SW3 osteocyte‐like cells. Whole body phosphate retention because of decreased renal function may be the direct physiological stimulus for FGF23 secretion.28 Our results showed no significant correlation between plasma phosphate and FGF23 concentrations. This has been reported previously in a study of geriatric non‐azotemic cats.12 In humans with normal kidney function, a correlation between urinary phosphate excretion and tubular reabsorption but not serum phosphate concentration has been identified.28 A rat model of early CKD identified increased circulating concentrations of FGF23, normal serum phosphate concentrations, and increased fractional excretion of phosphate, but when FGF23 activity was inhibited fractional excretion of phosphate decreased and serum phosphate concentrations increased, suggesting that in early CKD, normal plasma phosphate concentrations can be maintained by the response of functioning nephrons to increased circulating concentrations of FGF23.29
In humans, dietary phosphorus manipulation is an important determinant of serum FGF23 concentration independent of serum phosphate concentration, sex, age, and serum PTH concentration.30 Interestingly, chronic excessive consumption of highly available dietary phosphate may contribute renal injury and development of CKD.31 A recent observational study of 62 client‐owned cats showed a significantly higher dietary phosphate and protein content of the food fed before diagnosis in cats that developed CKD compared to controls.32 No detailed dietary information was available for the cats in our study, and therefore, the influence of dietary phosphate on plasma FGF23 concentration or association with early renal dysfunction and increased plasma SDMA concentrations could not be determined. Long‐term studies evaluating the effect of excess dietary phosphate on kidney function, plasma SDMA concentration, plasma FGF23 concentration, and renal handling of phosphate are needed to fully understand possible mechanisms of renal injury and the relationship between plasma FGF23 concentrations and renal handling of phosphate.
A number of other factors have been associated with circulating FGF23 concentration. A study of cats with naturally occurring CKD observed an inverse association between plasma tMg concentration and FGF23 concentration, and it has been hypothesized that FGF23 has an effect on renal magnesium handling.33 In our study, tMg was not significantly different between cats with increased SDMA concentrations and those with normal SDMA concentrations, and no significant correlation was found between plasma FGF23 and tMg concentrations. However, this association previously has only been found in azotemic CKD cats. Furthermore, in our study, plasma tMg concentrations were available for only 5 cats with increased SDMA concentrations and in 36 cats with plasma SDMA concentrations within the reference range, which would be considered to have normal renal function. Total magnesium concentration consists of protein‐bound, complexed, and ionized fractions. Although no consensus exists on whether plasma ionized or tMg measurement best represents magnesium status, a weak correlation has been found between ionized and total plasma magnesium concentration, and ionized magnesium status may be overestimated by tMg measurement.34
Serum iron concentration and systemic inflammation also have been found to have a role in regulation of FGF23.35 In a mouse model of acute inflammation, a marked increase in FGF23 messenger RNA expression and inactive cleaved c‐terminal FGF23 was observed, but intact active FGF23 (iFGF23) concentration remained unaltered, suggesting a role for inflammatory cytokines in regulation of osseous FGF23 production and cleavage. This finding is particularly interesting in the context of CKD in cats, where severe dental disease, which is associated with an inflammation, has been identified as a risk factor for the development of CKD.36 In human patients, low serum iron concentrations are associated with high concentrations of iFGF23 independent of markers for inflammation and renal function.37 A recent study in cats found significantly higher mean serum amyloid A and hepcidin concentrations and significantly lower mean total iron concentrations and total iron‐binding capacity in cats with CKD compared to healthy controls, suggesting that CKD in cats is associated with systemic inflammation and altered iron metabolism.38 In our study, PCV was not significantly different between groups but investigation into iron or inflammatory status was not performed. Future research including measurement of inflammatory cytokines to characterize the complex relationship among inflammation, iron deficiency as well as plasma phosphate and FGF23 concentrations in both healthy cats and those with CKD is indicated.
One potential limitation of our study is that measurement of plasma SDMA, PTH, and FGF23 concentrations did not occur for all samples at the time of sampling. Frozen plasma samples (approximately −80°C) were used for measurement of SDMA, FGF23 and PTH concentrations within 18 (FGF23 and PTH) or 70 (SDMA) months of the sample date, leading to the possibility that degradation overtime could affect the measured concentrations. Symmetric dimethylarginine concentrations have been shown to be stable over 14 days in canine plasma samples stored at 4°C and analyzed using liquid chromatography mass spectrometry.39 Similarly, the effect of storage time has been studied for PTH and FGF23, with no effect of storage seen on FGF23 concentrations in feline plasma stored in EDTA at −20°C for 14 days15 and on PTH concentrations in plasma samples stored in EDTA at −80°C for a mean of 50 days. It should be noted however that a now commercially unavailable immunoradiometric assay was used in this study.40 Stability of SDMA, FGF23, and PTH in plasma stored at −80°C for several years has not been investigated. However, studies in cats diagnosed with hyperthyroidism or CKD, in which FGF23 and PTH concentrations were measured retrospectively over a similar time frame as the current study, showed higher plasma FGF23 and PTH concentrations in samples taken before therapeutic interventions compared to post‐intervention samples, despite the longer storage time of the pre‐intervention samples, indicating that there was minimal effect of storage on measured FGF23 and PTH concentrations.15, 41 Furthermore, in our study, there was no evidence of a negative effect of storage time on plasma concentrations of FGF23, PTH, and SDMA (data not shown). Therefore, it seems probable that degradation of FGF23, PTH, and SDMA is not an important factor, but we cannot exclude the possibility that storage of samples had some impact in our study.
In conclusion, an early increase in circulating FGF23 has been reported in humans and cats with CKD. We observed that more cats with increased plasma SDMA concentration had increased circulating FGF23 concentrations compared to those with plasma SDMA concentrations within the reference range. This finding, together with our previous observations in geriatric pet cats that plasma FGF23 concentration was predictive of the onset of azotemia,12 suggests that a disturbance in phosphate homeostasis occurs in early stage CKD. Despite our study's limitations, these observations should be applicable to other populations of pet cats, having been undertaken on client‐owned cats with naturally occurring CKD. A greater understanding of phosphate homeostasis and the effect of dietary phosphate restriction in the pre‐azotemic stages of CKD in cats on disease progression and survival should allow earlier intervention and dietary treatment to be tailored to the patient's individual requirements. Circulating FGF23 concentration may have diagnostic utility in identification of those cats that require phosphate restriction in the non‐azotemic stage of CKD and in monitoring their response to treatment.
CONFLICT OF INTEREST DECLARATION
Hannah Sargent received PhD studentship funded by Royal Canin. Jonathan Elliott was a consultant in Elanco Ltd, CEVA Animal Health Ltd, Boehringer Ingelheim Ltd, Bayer Animal Health, Orion Incorp, Idexx Ltd, Nextvet Ltd, Waltham Centre for Pet Nutrition. Received grant funding from Royal Canin Ltd, Elanco Ltd, Waltham Centre for Pet Nutrition, Zoetis Ltd, CEVA Animal Health. Member of the International Renal Interest Society which receives a grant from Elanco Ltd.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was part of a larger observational cohort for which approval of the Ethics and Welfare Committee of the Royal Veterinary College had been granted.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Preliminary results of this study were presented at ECVIM Congress 2017, St Julian's, Malta. The Renal Research Clinic at the Royal Veterinary College acknowledges support from Royal Canin for its research on chronic kidney disease‐mineral and bone disorder in cats.
Sargent HJ, Jepson RE, Chang Y‐m, Biourge VC, Bijsmans ES, Elliott J. Fibroblast growth factor 23 and symmetric dimethylarginine concentrations in geriatric cats. J Vet Intern Med. 2019;33:2657–2664. 10.1111/jvim.15590
Funding information Royal Canin
REFERENCES
- 1. Kielstein JT, Salpeter SR, Bode‐Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function‐‐a meta‐analysis. Nephrol Dial Transplant. 2006;21(9):2446‐2451. [DOI] [PubMed] [Google Scholar]
- 2. Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l‐arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med. 2008;22(2):317‐324. [DOI] [PubMed] [Google Scholar]
- 3. Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med. 2014;28(6):1699‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014a;28(6):1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. IRIS Kidney . 2015. www.iris-kidney.com/guidelines/recommendations.html. Accessed July 23, 2017.
- 6. Liu S, Guo R, Simepson LG, et al. Regulation of fibroblast growth factor 23 but not degradation by PHEX. J Biol Chem. 2003;278(39):37419‐37426. [DOI] [PubMed] [Google Scholar]
- 7. Ranch D, Zhang MY, Portale AA, et al. Fibroblast growth factor 23 regulates renal 1,25‐dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice. J Bone Miner Res. 2011;26(8):1883‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD‐related mineral and bone disorder. Kidney Int Suppl. 2011;79(121):S3‐S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945‐1953. [DOI] [PubMed] [Google Scholar]
- 10. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentration in geriatric cats with various degrees of renal function. J Am Vet Med Assoc. 2012;241(10):1326‐1335. [DOI] [PubMed] [Google Scholar]
- 12. Finch NC, Geddes RF, Syme HM, Elliott J. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med. 2013;27(2):227‐233. [DOI] [PubMed] [Google Scholar]
- 13. Michel KE, Anderson W, Cupp C, Laflamme DP. Correlation of a feline muscle mass score with body composition determined by dual‐energy X‐ray absorptiometry. Br J Nutr. 2011;106:S57‐S59. [DOI] [PubMed] [Google Scholar]
- 14. Williams T, Elliott J, Syme H. Calcium and phosphate homeostasis in hyperthyroid cats‐ associations with development of azotemia and survival time. J Small Anim Pract. 2012;53(10):561‐571. [DOI] [PubMed] [Google Scholar]
- 15. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27(2):234‐241. [DOI] [PubMed] [Google Scholar]
- 16. Relford R, Roberson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin Small Anim Pract. 2016;46(6):941‐960. [DOI] [PubMed] [Google Scholar]
- 17. Finch NC. Measurement of glomerular filtration rate in cats: methods and advantages over routine markers of renal function. J Feline Med Surg. 2014;16(9):736‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marsell R, Grundberg E, Krajisnik T, et al. Fibroblast growth factor‐23 is associated with parathyroid hormone and renal function in a population‐based cohort of elderly men. Eur J Endocrinol. 2008;158(1):125‐129. [DOI] [PubMed] [Google Scholar]
- 19. Bacchetta J, Dubourg L, Harambat l, et al. The influence of fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metabol. 2010;95(4):1741‐1748. [DOI] [PubMed] [Google Scholar]
- 20. Wang Q, Su W, Shen Z, et al. Correlation between soluble α‐klotho and renal function in patients with chronic kidney disease: a review and meta‐analysis. Biomed Res Int. 2018;2018:9481475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018;19(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Razzaque MS. FGF23‐mediated regulation of systemic phosphate homeostasis: is klotho an essential player? AJP Renal Physiol. 2008;296(3):F470‐F476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82(7):737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pereira RC, Juppner H, Azucena‐Serrano CE, et al. Patterns of FGF‐23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Christov M, Waikar S, Pereira R, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84150(4):776‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander, J. , Butterwick, R. , Stockman, J. , et al. Effect of diets enriched with inorganic phosphorus on the adult feline kidney [online]. 2018. Submitted Manuscript. Available at: https://osf.io/ykbce/ [DOI] [PMC free article] [PubMed]
- 27. Saito H, Maeda A, Ohtomo S, et al. Circulating FGF23 is regulated by 1alpha, 25‐dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280(4):2543‐2549. [DOI] [PubMed] [Google Scholar]
- 28. Ferrari S, Bonjour R, Rizzoli R. Fibroblast growth factor 23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metabol. 2005;90(3):1519‐1524. [DOI] [PubMed] [Google Scholar]
- 29. Hasegawa, H. , Nageno, N. , Urakawa, I. , et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early‐stage chronic kidney disease. Kidney Int 2010;78(10):975‐980l [DOI] [PubMed] [Google Scholar]
- 30. Burnett S, Gunawardene S, Bringhurst F, et al. Regulation of C‐terminal and intact FGF23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187‐1196. [DOI] [PubMed] [Google Scholar]
- 31. Dobenecker B, Webel A, Reese S, Kienzle E. Effect of a high phosphorus diet on indicators of renal health in cats. J Feline Med Surg. 2018;20(4):339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boswald L, Kienzle E, Dobenecker B. Observations about phosphorus and protein supply in cats and dogs prior to the diagnosis of chronic kidney disease. J Anim Physiol Anim Nutr. 2018;102:31‐36. [DOI] [PubMed] [Google Scholar]
- 33. Van den Broek D, Chang Y, Elliott J, et al. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. J Vet Intern Med. 2018;32(4):1359‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johansson M, Whiss PA. Weak relationship between ionised and total magnesium in serum of patients requiring magnesium status. Biol Trace Elem Res. 2007;115(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 35. David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finch N, Syme H, Elliott J. Risk factors for development of chronic kidney disease in cats. J Vet Intern Med. 2016;30(2):602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewerin C, Ljunggren O, Nilsson‐Ehle H, et al. Low serum iron is associated with high serum FGF23 in elderly men: the Swedish MrOS study. Bone. 2017;98:1‐8. [DOI] [PubMed] [Google Scholar]
- 38. Javard R, Grimes C, Bau‐Gaudreault L, Dunn M. Acute‐phase proteins and iron status in cats with chronic kidney disease. J Vet Intern Med. 2017;31(2):457‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nabity MB, Lees GB, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29:1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barber PJ, Elliott J, Torrance AG. Measurement of feline intact parathyroid hormone: assay validation and sample handling studies. J Small Anim Pract. 1999;39:108‐116. [Google Scholar]
- 41. Williams TL, Elliott J, Syme H. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53(10):561‐571. [DOI] [PubMed] [Google Scholar]


