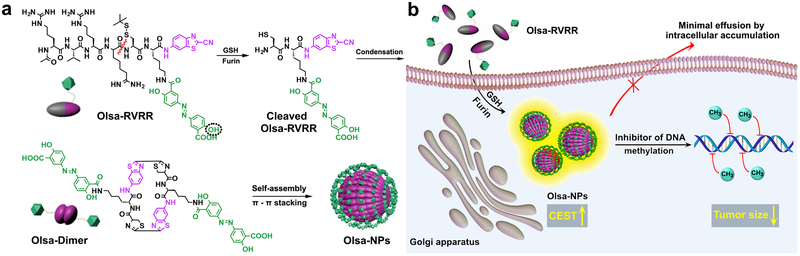
Fig. 1. Schematic illustration for the formation of Olsa-NPs by furin-mediated intracellular reduction and condensation of Olsa-RVRR, resulting in enhanced CEST signal and tumor treatment efficacy.
(a) Self-assembly of Olsa-RVRR into Olsa-NPs through a series of steps. Red line indicates the site of furin cleavage, and the dotted circled hydroxyl group indicates the exchangeable hydroxyl proton that provides OlsaCEST signal at 9.8 ppm from the water frequency. (b) After Olsa-RVRR enters the cytoplasm of furin-overexpressing cells (HCT116 cells in this study), it undergoes cleavage of the peptide by activated furin near the Golgi complex, where the reduction of disulfide by glutathione (GSH) generates cleaved Olsa-RVRR. Amphiphilic oligomers (mostly dimers) are then formed from the click reaction between two cleaved Olsa-RVRR molecules, followed by self-assembly into Olsa-NPs as a result of intermolecular π-π stacking. The intracellular accumulation of Olsa-NPs then serves as a reservoir of olsalazine molecule enhancing CEST contrast and inhibiting DNA methylation for tumor therapy.