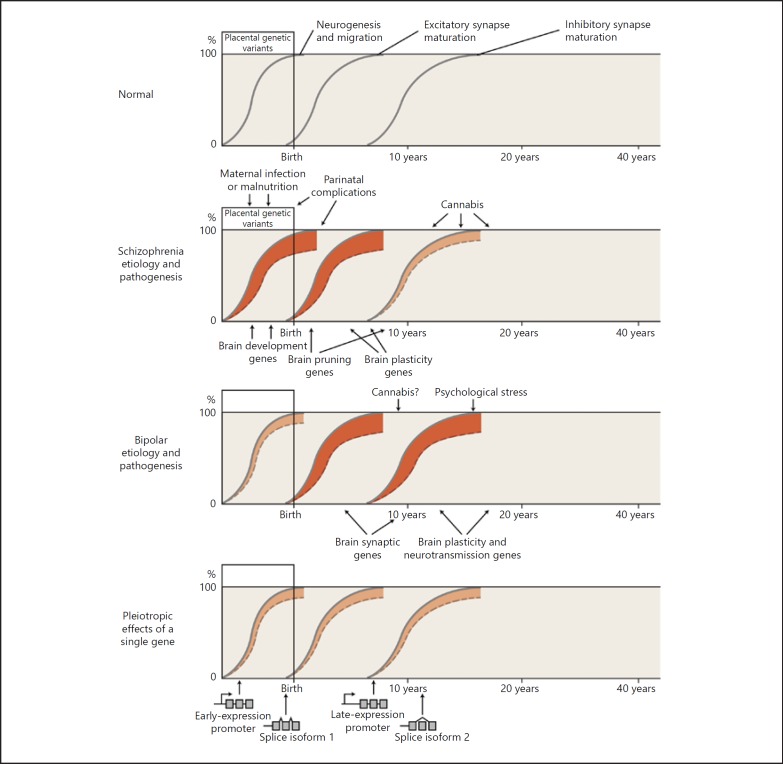
Fig. 5.
Relationship between genetic and environmental risk factors and clinical phenotypes. Genetic and environmental factors, acting in part on the differential expression of genes, disrupt the normal developmental processes, leading to abnormal brain structure and function. The y axis indicates the extent to which a developmental process has reached completion. The x axis indicates age, reflecting developmental status. A solid gray line indicates normal brain development (neurogenesis and migration, excitatory synapses, and inhibitory synapses). A dashed line below the solid gray line, with red shading between the lines, indicates a disruption of the process – a shift to an abnormal brain state (depiction modified from Insel [93]). Normal development involves multiple neurobiological processes; here we show just three as a simplification to highlight that different processes mature at different times. Phenotypic differences arise because of individual differences in exposure to genetic and environmental risk factors, in the timing of exposure to environmental risk factors, and in the interactions among genetic and environmental risk factors. We emphasize here that risk factor-induced changes in gene expression, including different isoforms of the same gene, may lead to changes in synaptic structure and function, though many other pathogenic mechanisms are also likely involved. We hypothesize (no doubt as an oversimplification) that risk alleles and environmental risk factors acting during earlier stages of development (such as placental stress occurring in an individual with a genetic vulnerability to such stress) tend to predispose to schizophrenia. By contrast, in general, alleles and environmental factors altering gene expression later in development predispose more to bipolar disorder. The earlier influences may preferentially affect excitatory synapses (which generally mature earlier), while the later influences may preferentially affect inhibitory synapses (which generally mature later). The bottom panel highlights how different alleles of the same gene can have effects at different time points in development via differing promotor usage or splice isoforms. Many other mechanisms (not shown), such as epigenetic changes or posttranslational regulation via microRNAs, could give similar results. We are well aware that these hypotheses involve oversimplifications and may turn out to be incorrect, but we believe that it is useful to develop and test concepts that attempt to correlate genetic, pathophysiological, and clinical information. Illustrator: Joan M.K. Tycko.