Abstract
Introduction
Near-infrared fluorescence imaging with indocyanine green is a useful tool during partial nephrectomy. Because an accurate method for judging hasn't been established yet, the success rate may be slightly different and inconsistent.
Materials and Methods
A total of 21 cases with suspected renal cancers who had undergone a partial nephrectomy were enrolled. We examined differences in the success rate between malignant lesions and the parenchyma by quantifying fluorescence in the pre-resection and ex vivo phases.
Results
Pre-resection imaging showed a significant degradation of fluorescence in the focused lesion in 76.2% (16/21) of cases. A significant degradation was observed in 73.7% (14/19) of the total malignant lesions, 70.5% (12/17) of cases with a clear cell lesion, 100% (2/2) of cases with non-clear cell lesions, and 100% (2/2) of benign angiomyolipomas. In contrast, imaging of the ex vivo resected specimens showed a significant degradation in fluorescence of the focused lesions in 85.7% (18/21) of cases. A significantly degradation was observed in 84.2% (16/19) of the total malignant lesions, 82.3% (14/17) of cases with a clear cell lesion, 100% (2/2) of cases with non-clear cell lesions, and 100% (2/2) of benign angiomyolipomas.
Conclusion
We firstly evaluated the efficacy of quantitative indocyanine green-based fluorescence as an objective method.
Keywords: Partial nephrectomy, Indocyanine green, Infrared fluorescence
Introduction
During partial nephrectomy for renal cancer, it is important to maintain a resected margin between the tumor lesion and normal lesion in order to achieve a curable surgical procedure. In general, the shape of the resection is established by pre-operative radiological imaging, peri-operative ultrasound imaging, and endoscopic or optical imaging with incandescent light. However, only a few times can achieve a positive surgical margin and therefore a navigation system is indispensable for determining the appropriate shape of the resection design. To date such a system has not been established. One system of navigation, near-infrared fluorescence imaging with indocyanine green (ICG), has been evaluated in other fields such as hepatectomy [1] and for detecting sentinel lymph nodes in prostatectomy [2]. For renal surgery, ICG navigation was shown to be the optimal tool for identifying the ischemic area [3], while transarterial superselective intrarenal mass delivery of ICG-lipiodol mixture was effective for identification and anatomical dissection [4]. In other aspect, the loss of fluorescence in renal cancers caused by functional impairment of bilitranslocase was reported in 2008 by Golijanin et al. [5]. This ICG-based fluorescence navigation system showed differences in color between tumors and normal lesions and provided helpful information for confirming a negative surgical margin determined by visual judgment in open surgery [6] and robotic-assisted surgery [7,8]. However, the definitive criteria for evaluating the efficacy of ICG-based fluorescence navigation remains uncertain, because the efficacy achieved was different in each study [9].
To determine the definitive criteria of efficacy we performed minimum incision endoscopic partial nephrecto-mies guided by an ICG-based fluorescence navigation system and compared the objective quantitation of fluorescence between tumor lesions and the parenchyma of the kidney using ImageJ software.
Materials and Methods
Cases with suspicious renal cancers who underwent a partial nephrectomy between July 2013 and December 2017 were recruited for this cohort study at Aichi Cancer Center Hospital. We enrolled 21 cases with suspicious renal cancers who underwent a partial nephrectomy, using minimal invasive open surgery that involved an endoscopy-guided minimal incision, as reported previously [10]. Before the operation, informed consent to use ICG-based fluorescence navigation was obtained from all the patients, except for those with an allergic history against iodide component, because this component may cause an allergic response against ICG [11]. The study was approved by our institutional review board.
ICG-Based Fluorescence Navigation
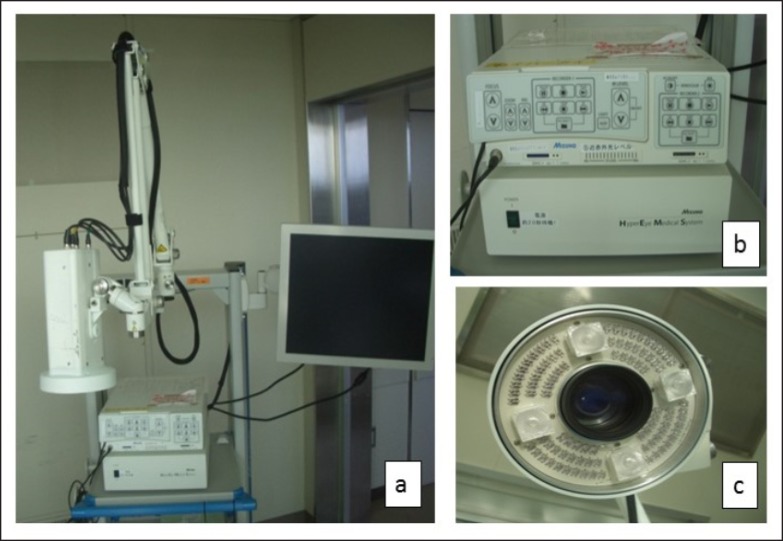
We prepared a 2.5mg/ml ICG solution (Diagnogreen® 25 mg, Daiichi-Sankyo Pharmaceutical, Tokyo, Japan). The HyperEye Medical System (Mizuho Co., Tokyo, Japan) was used as the near-infrared device (wavelength 800-850 nm) (Fig. 1a). This system provided illumination with near-infrared light of 760-780 nm (Fig. 1b). The camera unit of this system (Fig. 1c) enables visualization of invisible near-infrared rays on a monitor. ICG produced fluorescence reflected by near-infrared light at a wavelength of 800-850 nm.
Fig. 1.
Appearance of the HyperEye Medical System. a Overview of the HyperEye Medical System (Mizuho co.); b System for near-infrared fluorescent imaging; c Camera head used for fluorescent imaging.
ICG-Based Fluorescence Navigation-Guided Partial Nephrectomy
When we suspected there was a high possibility of the resection margin extending into the renal pelvic area, the main renal artery was clamped. A 2 ml aliquot (5 mg) of ICG solution was injected intravenously before clamping in these cases. On the other hand, when the limitation of the extended margin of resection was estimated, a microwave system (Microtaze, Alfresa Co., Osaka, Japan) was prepared, without clamping of the main renal artery. To avoid bleeding in some cases of the microwave system, the ICG solution was injected before use of the microwave and resection.
White colored fluorescence imaging appeared in the parenchyma area a few seconds after the ICG injection. The entire fluorescence images were recorded to estimate the quantity of fluorescence for the next step. The fluorescence imaging of the resected specimen was recorded within 1 hour after resection.
Estimated Quantitation of Fluorescence
A freeze-frame picture of a video image after 1 minute was captured, followed by an ICG injection. A picture of the resected specimen was recorded after 1 hour followed by resection.
The images were used for quantitation in the next stage. For quantitation of fluorescence, we used an application tool, ImageJ [12]. A freeze-frame picture was captured by ImageJ and the tumor and normal areas were traced separately. The histogram of each traced area was produced, followed by quantification of the amount of fluorescence.
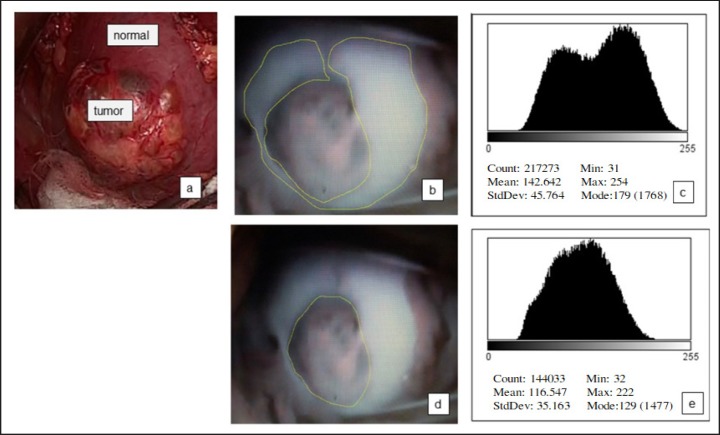
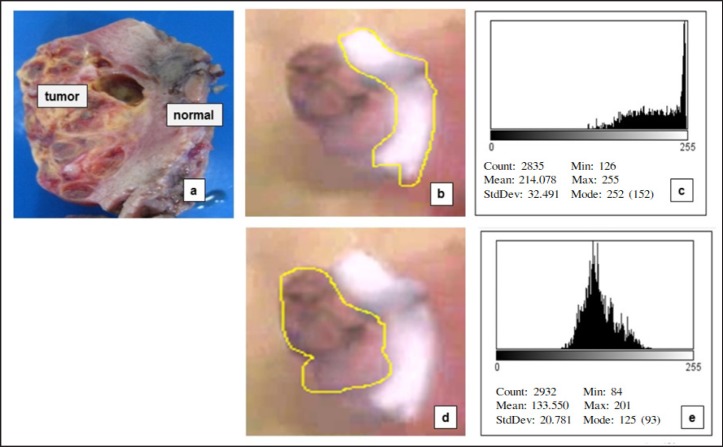
To explain the steps for quantifying fluorescence in detail, we have presented a significant case image of patient No. 2 (Fig. 2, 3). Pre-operative imaging (Fig. 2) and the ex vivo image (Fig. 3) were captured and the area of normal parenchyma (Fig. 2b, 3b) and tumor lesion (Fig. 2d, 3d) traced using the ImageJ program. A histogram of each area was prepared (Fig. 2c, e, 3c, e), followed by statistical analysis to quantify the amount of fluorescence. Significant degradation of fluorescence in the tumor lesion was determined by comparison with a normal lesion (p < 0.001).
Fig. 2.
Steps for quantifying fluorescence in the pre-operative image. a Appearance under the natural light before resection of the tumor; b Imaging under near-infrared fluorescence: trace of the normal area; c Histogram of the normal parenchyma; d Imaging under near-infrared fluorescence: trace of the tumor area; e Histogram of the tumor area.
Fig. 3.
Steps for quantifying the fluorescence in the image of the resected specimens. a Appearance of the resected specimen under natural light; b Imaging under near-infrared fluorescence: trace of the normal area; c Histogram of the normal parenchyma; d Imaging under near-infrared fluorescence: trace of the tumor area; e Histogram of the tumor area.
Statistical Analysis
The un-paired t test was used to compare the categorical variables. Differences with p < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 15 software (SPSS, Chicago, IL, USA).
Results
Characteristic of the Patients in the Study
The characteristics of the 21 patients in the study are described in Table 1. The distribution of the location of the lesions was 6 cases (28.6%) with an endophytic lesion, 13 cases (47.6%) with an exophytic lesion and 2 (9.4%) with a mixed lesion. In terms of surgical procedures, the microwave technique was used in 11 cases (52.4%) and the arterial clamping technique in 10 cases (47.6%).
Table 1.
Baseline characteristics of the patients in the study
| Patient demography | Values |
|---|---|
| Number of masses | 21 |
| Mean age, years (range) | 63.7 ± 10.2 (39–74) |
| Gender, n (%) | |
| Male | 16 (76.2%) |
| Female | 5 (23.8%) |
| Tumor site, n (%) | |
| Right | 9 (42.8%) |
| Left | 12 (57.2%) |
| Mean maximum diameter, cm (range) | 2.4 ± 0.9 (1.5–5.0) |
| Location, n (%) | |
| Endophytic | 6 (28.6%) |
| Exophytic | 13 (47.6%) |
| Mixed | 2 (9.4%) |
| Surgical procedure | |
| Microwave | 11 (52.4%) |
| Clamping | 10 (47.6%) |
| Mean warm ischemic time, minutes (range) | |
| Microwave | 24 (17–34) |
| Clamping | 0 |
| Benign total, n (%) | 2 (9.5%) |
| Angiomyolipoma | 2 (9.5%) |
| Malignancy total, n (%) | 19 (90.5%) |
| Clear cell | 17 (81.1%) |
| Papillary | 1 (4.7%) |
| Chromophobe | 1 (4.7%) |
The final pathological results revealed 2 cases (9.5%) of angiomyolipoma and 19 cases (90.5%) of malignant tumor. The absence of a positive surgical margin was accomplished in all cases with a partial nephrectomy. No case with an allergy against ICG was recorded.
Estimated Quantitation of Fluorescence
Table 2 provides a summary of quantitation of fluorescence, background of tumor shape, surgical technique, and pathology in all the patients. Pre-operative imaging demonstrated a significant degradation in fluorescence of the focused lesion in 16 (76.2%) cases, but not in the remaining 5 cases (23.8%).
Table 2.
Results of quantitation of fluorescence
| Case | Shape | Surgical procedure | Pathology | Pre-respective condition |
Resected specimen inex vivo |
||||
|---|---|---|---|---|---|---|---|---|---|
| Normal lesions | Tumor lesions | Value | Normal lesions | Tumor lesions | Value | ||||
| 1 | exophytic | microwave | clear | 162.7 ± 25.4 | 129 ± 40.9 | significant | 154.1 ± 11.4 | 94.4 ± 25.4 | significant |
| 2 | exophytic | microwave | clear | 142.6 ± 45.6 | 116.5 ± 35.1 | significant | 214.1 ± 32.5 | 133.6 ± 20.8 | significant |
| 3 | exophytic | microwave | clear | 175.3 ± 31.6 | 193.1 ± 29.1 | not significant | 115.0 ± 16.4 | 118.3 ± 9.5 | not significant |
| 4 | exophytic | clamping | clear | 212.0 ± 21.5 | 192.0 ± 23.7 | not significant | 32.9 ± 6.9 | 7.5 ± 28.0 | not significant |
| 5 | endophytic | microwave | clear | 166.6 ± 36.2 | 121.1 ± 35.1 | significant | 132.8 ± 14.2 | 65.1 ± 19.3 | significant |
| 6 | endophytic | clamping | clear | 202 ± 25.1 | 148.5 ± 12.2 | significant | 150.0 ± 13.3 | 90.4 ± 11.8 | significant |
| 7 | exophytic | microwave | clear | 97.6 ± 8.9 | 90.5 ± 15.4 | not significant | 168.7 ± 21.1 | 97.7 ± 18.6 | not significant |
| 8 | exophytic | clamping | clear | 160.5 ± 13.6 | 79.2 ± 7.1 | significant | 171.0 ± 17.2 | 195.0 ± 5.2 | significant |
| 9 | endophytic | clamping | AML | 124.7 ± 20.5 | 95.2 ± 11.0 | significant | 91.3 ± 17.0 | 48.7 ± 8.1 | significant |
| 10 | endophytic | clamping | AML | 82.2 ± 9.0 | 50.7 ± 8.0 | significant | 202 ± 12.9 | 136.8 ± 9.6 | significant |
| 11 | endophytic | clamping | clear | 216.0 ± 24.1 | 143.0 ± 18.7 | significant | 186.8 ± 10.9 | 139.6 ± 20.7 | significant |
| 12 | exophytic | microwave | clear | 207.7 ± 15.9 | 172.0 ± 30.5 | significant | 111.5 ± 12.5 | 55.7 ± 18.3 | significant |
| 13 | exophytic | microwave | clear | 143.6 ± 29.3 | 103.0 ± 40.2 | significant | 171.9 ± 18.8 | 144.6 ± 13.0 | significant |
| 14 | exophytic | microwave | papillary | 133.1 ± 20.7 | 113.0 ± 8.2 | significant | 219.8 ± 11.1 | 169.4 ± 12.6 | significant |
| 15 | exophytic | microwave | clear | 210.5 ± 22.8 | 161.4 ± 9.0 | significant | 143.7 ± 19.0 | 94.6 ± 24.6 | significant |
| 16 | exophytic | clamping | chromophobe | 153.7 ± 31.5 | 55.3 ± 17.0 | significant | 139.0 ± 22.7 | 87.8 ± 17.6 | significant |
| 17 | endophytic | clamping | clear | 205.0 ± 34.8 | 124.2 ± 19.2 | significant | 120.4 ± 27.3 | 69.4 ± 13.8 | significant |
| 18 | exophytic | microwave | clear | 177.4 ± 30.7 | 114.2 ± 38.9 | significant | 236.4 ± 22.2 | 127.9 ± 34.1 | significant |
| 19 | exophytic | clamping | clear | 32.3 ± 11.0 | 39.7 ± 13.2 | not significant | 68.8 ± 21.9 | 40.5 ± 16.3 | not significant |
| 20 | mixed | clamping | clear | 191.3 ± 17.4 | 198.5 ± 23.2 | not significant | 109.6 ± 10.1 | 138.0 ± 13.3 | not significant |
| 21 | mixed | microwave | clear | 115.7 ± 20.3 | 33.9 ± 22.3 | significant | 118.6 ± 18.6 | 31.4 ± 33.0 | significant |
A comparison based on the shape of the focused lesions showed significant degradation in 100% (6/6) of endophytic cases, but only 66.7% (10/15) of exophytic or mixed types. With regard to the surgical procedures, significant degradation was observed in 81.8% (8/11) of cases who received the microwave system, and 70% (7/10) who had the procedure with clamping.
Based on the pathological results, significant degradation was found in 73.7% (14/19) of cases with total malignant lesions, 70.5% (12/17) of cases with clear cell lesions, 100% (2/2) of cases with non-clear cell lesions, and 100% (2/2) with a benign angiomyolipoma.
In contrast, imaging of the ex vivo resected specimens demonstrated significant degradation of fluorescence of the focused lesion in 18 (85.7%) cases, but not in 3 cases (14.3%). Comparison based on the shape of the focused lesions showed significant degradation in 100% (6/6) of endophytic cases, but only in 80% (12/15) of exophytic or mixed types. With regard to surgical procedures, significant degradation occurred in 90.9% (10/11) of cases with microwave surgery and 80% (8/10) of those who had clamping of the main renal artery. Comparison of pre-op-erative imaging and the resected specimens showed no difference in the degree of degradation in fluorescence.
The pathological results showed significant degradation in 84.2% (16/19) of cases with a total malignant lesion, 82.3% (14/17) of cases with a clear cell lesion, 100% (2/2) of cases with a non-clear cell lesion, and 100% (2/2) with a benign angiomyolipoma.
Discussion
The success rate for differentiating tumors from the parenchyma was reported to range between 73 and 88% [9]. Because an accurate method for this differentiation has not been established, the success rate for each case is slightly different and inconsistent. On the basis of this background, we firstly evaluated objective quantitation of fluorescence using ImageJ. Pre-operative imaging showed significant degradation in fluorescence of tumor lesions in 16 of 21 cases (76.2%), while imaging of the resected specimens demonstrated significant degradation of fluorescence in tumor lesions in 18 of 21 cases (85.7%). This result was similar to the lower rate reported in previous studies, while the rate in resected specimen was approximately equal to the higher rate seen in earlier reports [9]. We therefore attempted to determine the reason for the instability observed with ICG-based fluorescence navigation.
The first reason for this discrepancy was uncertainties regarding the method for evaluating the efficacy of ICG-based fluorescence navigation. In all previous reports, efficacy was influenced by subjective judgment, and also the categories of the lesion were either Grade 2 [13] or Grade 3 [6,14,15]. Because objective evaluation may be the ideal method, in the future it is necessary to restrict the method of estimation to objective quantitation and evaluate using the same methods for determining efficacy.
The second reason for the discrepancy was related to the dose of ICG. It is possible that an overdose of ICG may cause all tissue to fluoresce. However, to date a standardized dosing regimen has not been defined [8]. According to our results, using the same dose of ICG (5 mg), the degree of fluorescence in both pre-operative imaging and the resected specimen was quite different in each case. In the future, because the dose of ICG is a key factor for quantifying fluorescence, an optimal dosing regimen of ICG is needed to determine the difference between normal and tumor lesions.
The third reason for the discrepancy depended on differences in the pathological results. In general, the mechanism contributing to differences in fluorescence of ICG remains uncertain. One hypothesis is that the difference in fluorescence is due to variations in the transport of ICG into proximal tubule cells of normal renal parenchyma and poor retention of ICG in cancerous cells that contribute to hypofluorecence of cancer components [5]. A second hypothesis is that differences in the activity of the carrier protein, bilitranslocase in normal lesions may be a key factor for altering fluorescence [5]. Therefore, we suspect that compared with normal parenchyma, the differences may be attributable to alterations in the cell structure of focused lesions that are dependent on the pathological structure. To investigate the validity of that hypothesis, we compared the peri-operative rate of hypo-fluorecence in target lesions with that in the parenchyma based on our pathological results, and those reported that in malignant non-clear cells with solid chromophobe and papillary components, all the cases had differences between focused lesions and the parenchyma, but inconsistent results in clear cells and benign lesions [13,15,16,18] (Table 3). Based on these findings, the pattern of ICG fluorescence was not useful for predicting malignant or benign lesions [13]. In addition, the rate of pathological distribution has been shown to be related to ethnic background [17]. As shown in Table 3 (percentage of malignant 68.5-90.5%), our summary indicates the pathological distribution contributed to differences in the success rate of differentiating tumors from the parenchyma in all the cases. In the future, a molecular approach will be indispensable for determining the mechanism that results in different fluorescence under the same pathological conditions.
Table 3.
Summary of the peri-operative success rate of the target lesions with hypofluorecence compared with the parenchyma, based on the pathological results
| Author | Journal (published year) | % of malignant in total cases | Total | Clear cell carcinoma | Malignant pathological results |
Benign pathological results |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromophobe | Papillary | Cystic clear cell | Others | Oncocytoma | Angiomyolipoma | Cyst | Others | |||||
| Tobis et al. | Urology | 82.3% | 94.1% | 100% | 100% | 100% | 100% | 100% | 0% | |||
| [16] | (2012) | (14/17) | (16/17) | (8/8) | (1/1) | (4/4) | (1/1) | (2/2) | (0/1) | |||
| Tobis et al. | J Endurol | 68.5% | 68.4% | 100% | 100% | 0% | 0% | 0% | ||||
| [15] | (2012) | (13/19) | (13/19) | (10/10) | (3/3) | (0/1) | (0/2) | (0/3) | ||||
| Angell et al. | J Urol | 78.9% | 85.5% | 83.3 | 54.4% | 66.6% | 100% | |||||
| [8] | (2013) | (60/76) | (65/76) | (55/60) | (6/11) | (2/3) | (2/2) | |||||
| Manny et al. | J Endurol | 77.0% | 75% | 95.8% | 100% | 100% | 0% | 0% | 100% | 0% | 0% | 0% |
| [13] | (2013) | (77/100) | (75/100) | (46/48) | (6/6) | (13/13) | (0/7) | (0/3) | (8/8) | (0/8) | (0/5) | (0/2) |
| Our study | 2018 | 90.5% | 76.1% | 70.5% | 100% | 100% | 100% | |||||
| (19/21) | (16/21) | (12/17) | (1/1)) | (1/1) | (2/2) | |||||||
The fourth reason for the discrepancy was dependent on the effect induced by the position of the focused lesions. For the position of the tumor, differences between the tumor and normal parenchyma were easier to distinguish for more exophytic tumors than for endophytic tumors. And once the resection had commenced, the hypofluorescent nature of tumor will be appreciated better [15]. We evaluated the efficacy of ICG-based fluorescence navigation depending on the shape of the focused lesion. Based on the shape of the focused lesion, significant degradation was observed in all endophytic cases, but only in 66.7% (10/15) of exophytic or mixed types. Therefore the endophytic location may not be a disadvantage for determining efficacy, whereas the fluorescence of focused lesions may affect the efficacy of ICG-based fluorescence navigation.
What is the beneficial outcome of ICG-based fluorescence navigation? Because outcome may be influenced by two crucial points, the phase of the resection and evaluation of the ex vivo surgical margin, it is necessary to separately evaluate the efficacy of ICG-based fluorescence navigation during each phase. According to a previous report, ICG-based fluorescence navigation contributes to a reduction in warm ischemia time in the resection phase, but does not improve negative surgical margin or decreased complication in the ex vivo surgical margin [14].
Regarding evaluation during resection, easier handing of the camera in ICG-based fluorescence navigation should be indispensable, especially when switching from white light to the fluorescence image. The main advantage of the advanced system is that surgeons can switch from a standard white light to fluorescence imaging in the surgical console [9]. In contrast, the HyperEye Medical System is inappropriate for monitoring during resections due to less flexibility of the camera head and design of the structure itself. Therefore, improvements in the handling of the camera head will be needed in the future.
In general, evaluation of ex vivo lesions showed a better rate of diffraction (100%, 16/16) than that measured in pre-resected tumors (62.5%, 10/16) [6]. However, it is necessary to discuss the effects in normal parenchyma induced by microwaves, because no study has evaluated the effectiveness of ICG fluorescence in partial nephrec-tomy using microwaves, while the effects of microwaves on ICG fluorescence remain uncertain.
In general, microwaves cause coagulation in both sides of the parenchymal structure at the margins of the resection. Our results show that because of the alternation in color of both surfaces of the resected specimen due to coagulation, the stability of the homogeneous color of the resected specimen evaluated by optical judgment was insufficient to evaluate the remaining surgical margin. Accordingly, another method that optimizes evaluation of the resection margins needs to be developed.
In our study, we firstly evaluated whether the damage of normal parenchyma caused by microwaves was ignored or not. Our results showed the rate of significant degradation was 81.8% (8/11) and 90.9% (10/11) in cases before and after the use of microwaves, respectively. This finding indicates that the damage induced by microwaves in the normal parenchyma may be ignored, thus, ICG fluorescence will therefore be a useful tool for estimating the surgical margin in cases using the microwave system.
However, because the basic rate of a positive surgical margin during partial nephrectomy is extremely low [9], it is difficult to show an advantage by focusing on the degradation of a positive surgical margin.
In terms of management of safe use of ICG, the compound has the potential to induce an allergic reaction, because there is evidence that systemic reactions to ICG may be attributable to iodide components [11]. In fact, anaphylactic shock in response to intravenous ICG during a robotic partial nephrectomy has been reported [18]. In our study, a case with a history of an allergic reaction against iodide components was withdrawn from the study and therefore fortunately no severe adverse events have occurred. Therefore, it is important for future studies to pay attention to allergic events against ICG.
The main limitation of our study was its retrospective nature and the relatively small number of cases.
Unfortunately, the system for evaluating quantitative fluorescence imaging by ImageJ could not be provided as real time information, but only post-operative information. As a consequence, real time assessment of margin status could not be established using this version.
However, we were able to evaluate the efficacy of ICG-based fluorescence navigation using an objective quantitative method. We started the project using the same objective method to evaluate the robotic-assisted procedure, including the customized optimal dose of ICG for navigation. For the future, if the quantitation system using ImageJ is established as a real time assessment, this would provide surgeons with helpful information to estimate the status of the margin from 2 aspects. Firstly, in the pre-operative image, the design of the resection could be decided by quantitative changes in the area of fluorescence. Secondly, for post resection images, if stable quantitation of fluorescence in both surfaces of the resected specimens and parenchymal side is estimated, this will allow surgeons to confirm whether complete resection had been achieved.
References
- 1.Ishizawa T, Fukushima N, Shibahara J, Ma-suda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M, Kokudo N. Real-time identification of liver cancers by using indo-cyanine green fluorescent imaging. Cancer. 2009;115:2491–2504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 2.Manny TB, Patel M, Hemal AK. Fluorescence-enhanced robotic radical prostatectomy using real-time lymphangiography and tissue marking with percutaneous injection of unconjugated indocyanine green: the initial clinical experience in 50 patients. Eur Urol. 2014;65:1162–1168. doi: 10.1016/j.eururo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Harke N, Schoen G, Schiefelbein F, Heinrich E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: a single-surgeon matched-pair study. World J Urol. 2014;32:1259–1265. doi: 10.1007/s00345-013-1202-4. [DOI] [PubMed] [Google Scholar]
- 4.Simone G, Tuderti G, Anceschi U, Ferriero M, Costantini M, Minisola F, Vallati G, Pizzi G, Guaglianone S, Misuraca L, Gallucci M. “Ride the Green Light”: Indocyanine green-marked off-clamp robotic partial nephrec-tomy for totally endophytic renal masses. Eur Urol. 2019;75:1008–1014. doi: 10.1016/j.eururo.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Golijanin DJ, Marshall J, Cardin A, Singer EA, Wood RW, Reeder JE, Wu G, Yao JL, Passamonti S, Messing EM. Bilitranslocase (BTL) is immunolocalized in proximal and distal renal tubules and absent in renal cortical tumors using intravenous indocyamine green (ICG) (abstract) J Urol. 2008;179((suppl 4)):137. [Google Scholar]
- 6.Mitsui Y, Shiina H, Arichi N, Hiraoka T, In-oue S, Sumura M, Honda S, Yasumoto H, Igawa M. Indocyanine green (ICG)-based fluorescence navigation system for discrimination of kidney cancer from normal parenchyma: application during partial nephrec-tomy. Int Urol Nephrol. 2012;44:753–759. doi: 10.1007/s11255-011-0120-x. [DOI] [PubMed] [Google Scholar]
- 7.Tobis S, Knopf J, Silvers C, Yao J, Rashid H, Wu G, Golijanin D. Near infrared fluorescence imaging with robotic assisted laparo-scopic partial nephrectomy: initial clinical experience for renal cortical tumors. J Urol. 2011;186:47–52. doi: 10.1016/j.juro.2011.02.2701. [DOI] [PubMed] [Google Scholar]
- 8.Angell JE, Khemees TA, Abaza R. Optimization of near infrared fluorescence tumor localization during robotic partial nephrec-tomy. J Urol. 2013;190:1668–1673. doi: 10.1016/j.juro.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 9.Bjurlin MA, McClintock TR, Stifelman MD. Near-infrared fluorescence imaging with in-traoperative administration of indocyanine green for robotic partial nephrectomy. Curr Urol Rep. 2015;16:20. doi: 10.1007/s11934-015-0495-9. [DOI] [PubMed] [Google Scholar]
- 10.Soga N, Kato M, Masui S, Nishikawa K, Hasegawa Y, Yamada Y, Kise H, Arima K, Sugimura Y. Comparison of radical nephrec-tomy techniques in one center: minimal incision portless endoscopic surgery versus laparoscopic surgery. Int J Urol. 2008;15:1018–1021. doi: 10.1111/j.1442-2042.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 11.Bjerregaard J, Pandia MP, Jaffe RA. Occurrence of severe hypotension after indocya-nine green injection during the intraoperative period. A A Case Rep. 2013;1:26–30. doi: 10.1097/ACC.0b013e3182933c12. [DOI] [PubMed] [Google Scholar]
- 12.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 13.Manny TB, Krane LS, Hemal AK. Indocya-nine green cannot predict malignancy in partial nephrectomy: histopathologic correlation with fluorescence pattern in 100 patients. J Endourol. 2013;27:918–921. doi: 10.1089/end.2012.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krane LS, Manny TB, Hemal AK. Is near infrared fluorescence imaging using indo-cyanine green dye useful in robotic partial nephrectomy: a prospective comparative study of 94 patients. Urology. 2012;80:110–116. doi: 10.1016/j.urology.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 15.Tobis S, Knopf JK, Silvers C, Messing E, Yao J, Rashid H, Wu G, Golijanin D. Robot-assisted and laparoscopic partial nephrectomy with near infrared fluorescence imaging. J Endourol. 2012;26:797–802. doi: 10.1089/end.2011.0604. [DOI] [PubMed] [Google Scholar]
- 16.Tobis S, Knopf JK, Silvers CR, Marshall J, Cardin A, Wood RW, Reeder JE, Erturk E, Madeb R, Yao J, Singer EA, Rashid H, Wu G, Messing E, Golijanin D. Near infrared fluorescence imaging after intravenous indo-cyanine green: initial clinical experience with open partial nephrectomy for renal cortical tumors. Urology. 2012;79:958–964. doi: 10.1016/j.urology.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Soga N, Nishikawa K, Takaki H, Yamada Y, Arima K, Hayashi N, Sugimura Y. Low incidence of benign lesions in resected suspicious renal masses greater than 2 cm: single-center experience from Japan. Int J Urol. 2012;19:729–734. doi: 10.1111/j.1442-2042.2012.03030.x. [DOI] [PubMed] [Google Scholar]
- 18.Chu W, Chennamsetty A, Toroussian R, Lau C. Anaphylactic shock after intravenous administration of indocyanine green during robotic partial nephrectomy. Urol Case Rep. 2017;12:37–38. doi: 10.1016/j.eucr.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]