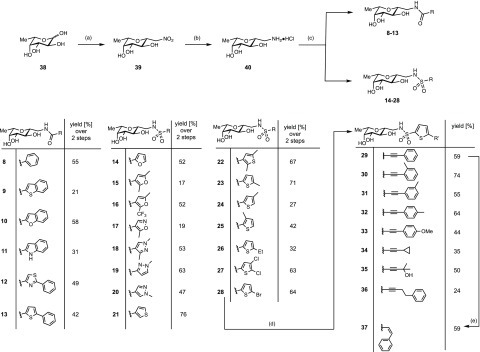
Scheme 1. Synthesis of the Amides 8–13 and Sulfonamides 14–38.
Reagents and conditions: (a) MeNO2, DBU, molecular sieves 3 Å, 1,4-dioxane, 50 °C, 3 d; (b)Pt/C, H2, HCl, MeOH, rt, 2 d; (c) acyl/sulfonyl chloride or carboxylic acid/EDC·HCl, Et3N, DMF, 0 °C; (d) CuI, Pd(PPh3)2Cl2, RCCH, Et3N, DMF, 50 °C, 16–42 h; (e) 1 atm H2, Lindlar’s catalyst, quinoline, rt, 46 h. Yields for 8–28 are given over two steps from the nitro derivative 39.