Figure 5.
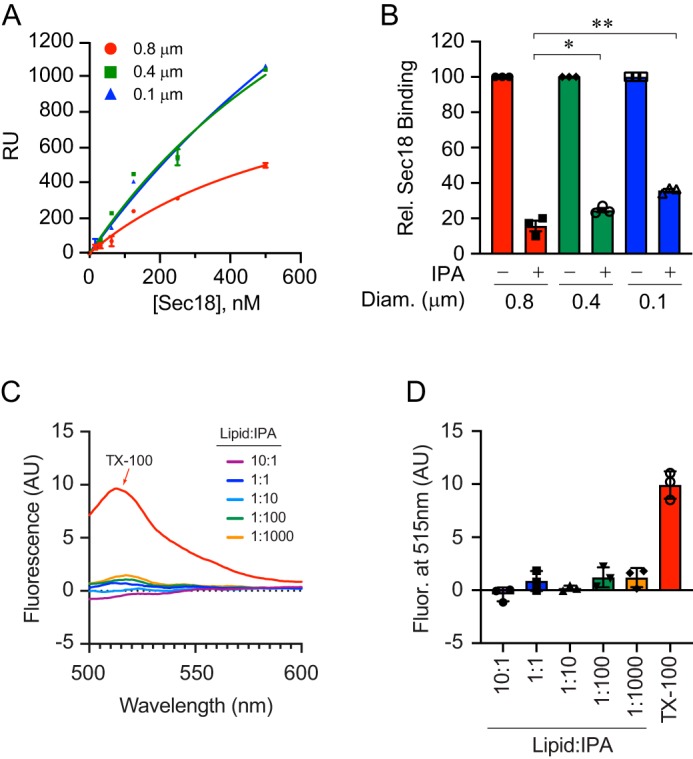
Liposome diameter affects IPA inhibition of Sec18 binding to PA. A, extruded PA liposomes of 0.1, 0.4, and 0.8 μm were bound to L1 biosensor chips. Sec18 was titrated to give a with KD 1600 ± 933, 1020 ± 549, and 673 ± 239 nm for 0.1-, 0.4-, and 0.8-μm diameter liposomes, respectively. Error bars represent S.E. (n = 3). B, as in A, Sec18 was flowed over PA liposomes attached to L1 chips in the presence or absence of 100 μm IPA. The difference in RU of Sec18 binding to liposomes in the presence of IPA was calculated relative to the maximum Sec18 bound in the absence of IPA. The bar graph represents the mean values of inhibition with individual points shown. The error bars represent S.E. (n = 3). C and D, liposome integrity in the presence of IPA was tested by calcein release. PA liposomes were extruded in the presence of 100 mm calcein, a concentration that leads to fluorescence quenching. Calcein containing liposomes were incubated with buffer, Triton X-100, or a dosage curve of IPA. Liposome damage, as measured by content leakage, was detected by the dilution of calcein and gain in fluorescence.
