Figure 1.
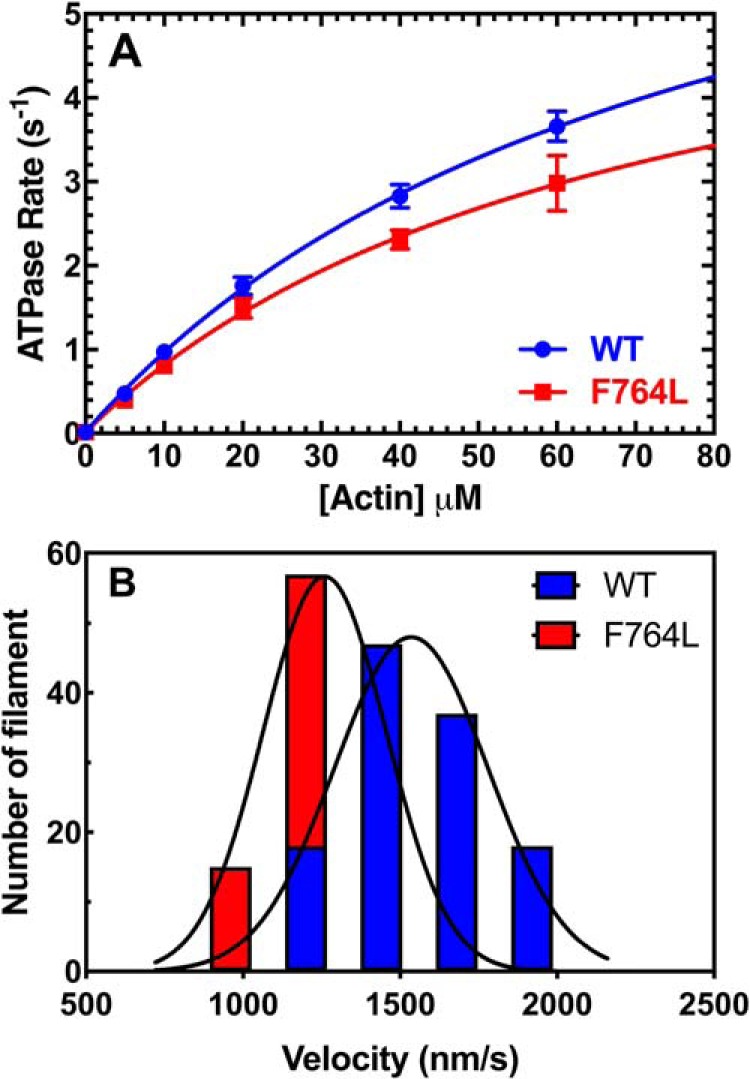
Steady-state ATPase and in vitro motility. A, the actin-activated ATPase activity of purified M2β-S1 WT and F764L in MOPS 20 buffer at 25 °C was determined as a function of actin concentration. The data at each actin concentration represents the average ± S.E. from 5 to 6 separate preparations. B, the sliding velocity of M2β-S1 WT and F764L in MOPS 20 buffer was analyzed manually via ImageJ. 120 filaments from 4 different protein preparations (30 filaments from each, 0.24–0.4 μm M2β-S1 loading concentration) were pooled together and fit to the Gaussian function. The average velocity is 1547 ± 19 nm/s for WT, and 1300 ± 17 nm/s for F764L.
