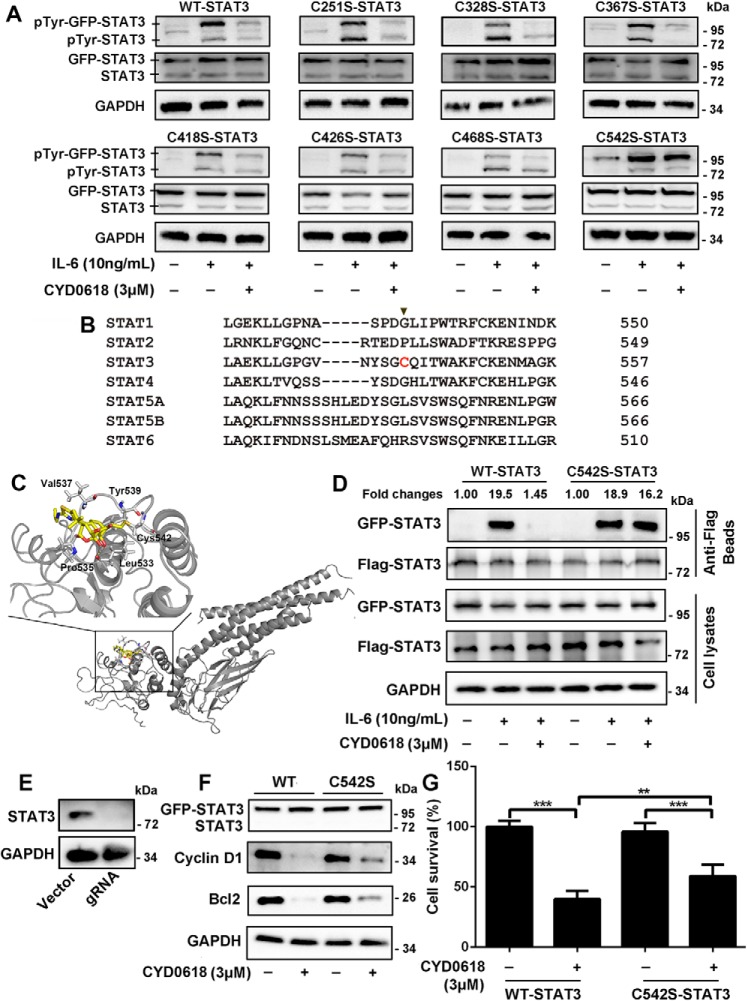
Figure 5.
Cysteine 542 of STAT3 is critical for the inhibition of STAT3 by CYD0618. A and B, HeLa cells were transfected with plasmids encoding the GFP-tagged WT, C251S, C328S, C367S, C418S, C426S, C468S, and C542S mutation of STAT3 for 24 h, respectively. The cells were then pre-treated with CYD0618 (3 μm) for 2 h before stimulation with IL-6 (10 ng/ml) for 15 min. Whole-cell lysates were processed for Western blot analysis with the indicated antibodies. B, sequence comparison of all human STATs proteins showing that the Cys-542 of STAT3 is unique (highlighted in red). C, molecular docking of the interaction between CYD0618 and STAT3. The crystal structure of STAT3 was obtained from PDB (code 1BG1). The covalent bond between the α,β-unsaturated carbonyl and the thiol of Cys-542 and the hydrogen bond between CYD0618 and Leu-533 are indicated. D, HeLa cells transfected with FLAG and GFP-tagged WT–STAT3 or C542S–STAT3 vectors were pre-treated with CYD0618 for 2 h, followed by stimulating with IL-6 (10 ng/ml) for 30 min. The cell extracts were subjected to immunoprecipitation, and immunoblotted with the indicated antibodies. E, HeLa cells were transfected with CRISPR–Cas9 plasmids targeting STAT3 or empty vector (vector). Endogenous protein levels in the polyclonal population were analyzed by immunoblotting. F and G, STAT3–knockout HeLa cells were transfected with GFP–WT–STAT3 vector or GFP–C542S–STAT3 vector for 24 h, followed by CYD0618 treatment for 24 h. Cells were then lysed and applied to immunoblotting with FLAG antibody and immunoblotted with the indicated antibodies (E) or measured by SRB staining (F). Data are expressed as mean ± S.D., n = 6. **, p < 0.01; and ***, p < 0.001 versus control.