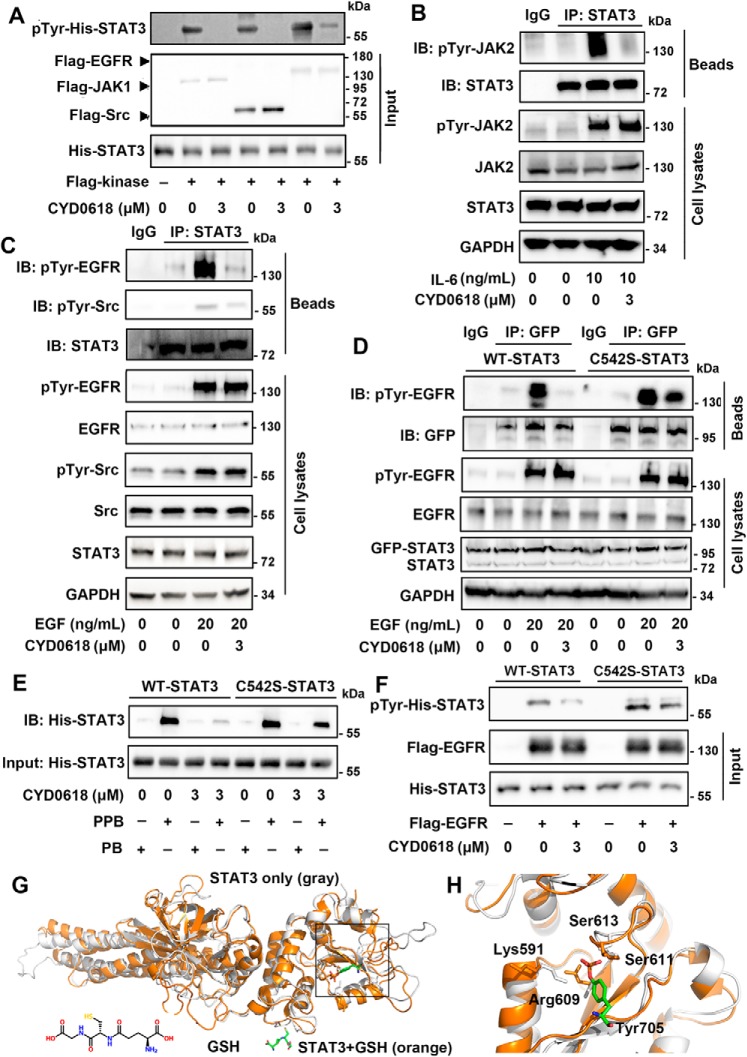
Figure 6.
CYD0618 disturbs the function of STAT3–SH2 domain via covalent modification of Cys-542. A and F, recombinant His–WT–STAT3 or His–C542S–STAT3 was treated with DMSO or CYD0618 for 1 h at room temperature. The mixtures were then incubated with the indicated purified FLAG kinases for 1 h at room temperature. Immunoblotting (IB) using the pTyr-705 STAT3 antibody reflects the effects of CYD0618 on kinase-induced phosphorylation of WT–STAT3 (A) or C542S–STAT (F) in vitro. B and C, CYD0618 inhibits the association of upstream kinases and STAT3. A2780 cells were pre-treated with CYD0618 (3 μm) for 2 h before stimulation with IL-6 (10 ng/ml) or EGF (20 ng/ml) for 30 min. Afterward, the whole-cell extracts were subjected to immunoprecipitation (IP) and immunoblotted with the indicated antibodies. D, C542S mutation regains the association of EGFR and STAT3. HeLa cells transfected with WT–STAT3 or C542S–STAT3 vector were pre-treated with CYD0618 for 2 h, followed by incubation of EGF (20 ng/ml) for 30 min. The whole-cell lysates were subjected to immunoprecipitation and immunoblotted with the indicated antibodies. E, effects of CYD0618 on the binding of Ac-pYLPQTV-NH2 to WT–STAT3 or C542S–STAT3 using the pulldown assay. Following incubation of CYD0618 for 1 h at room temperature, the recombinant His–WT–STAT3 or His–C542S–STAT3 was incubated with biotinylated Ac-pYLPQTV-NH2 (PPB) or biotinylated Ac-YLPQTV-NH2 (PB) and streptavidin beads for 1 h at room temperature. The mixtures were then subjected to immunoblotted with His antibody. G, representative global view of the GSH-mediated allosteric effect of STAT3 protein (PDB code 4E68). STAT3 protein alone is shown by the gray cartoon model; GSH–STAT3 complex is shown by the orange model. The disulfide bond between GSH and Cys-542 is identified and indicated by yellow line. H, representative view of the conformation change of the SH2 domain in STAT3 protein. The STAT3 protein alone is shown in gray, and GSH–STAT3 complex is shown in orange. The critical residues Lys-591, Arg-609, Ser-611, and Ser-613 are identified and indicated. The interaction between these two residues and pTyr-705 (stick model) of other STAT3 protein is also indicated.