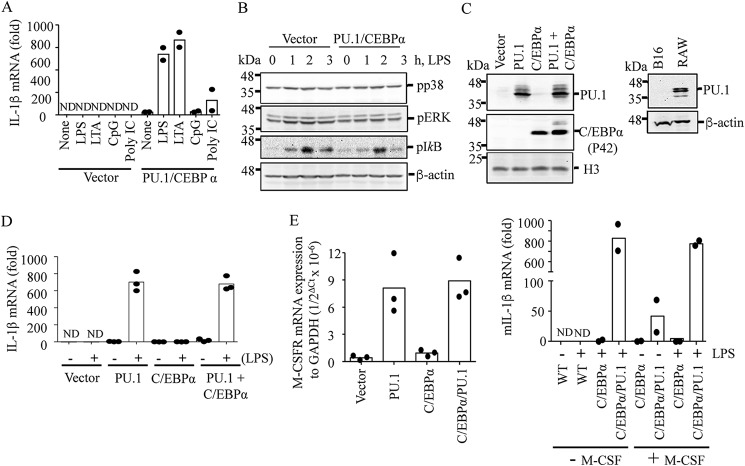
Figure 1.
Ectopic expression of PU.1 reprograms B16 cells to express IL-1β mRNA in response to TLR activation. B16 cells were transfected with pcDNA3 (Vector) or pcDNA3-HA-PU.1 (PU.1) and pMSCV-C/EBPα (CEBPα) for 24 h as described under “Experimental procedures.” A, after replating and culturing in a fresh dish for an additional 16–18 h, cells were stimulated with TLR4, -2, -9, and -3 ligands LPS (100 ng/ml), LTA (10 μg/ml), mouse CpG (20 μg/ml), and poly(I:C) (10 μg/ml), respectively, for 6 h. IL-1β mRNA transcription was then measured using RT-qPCR. Data are expressed as means ± S.D. (n = 2). B, cells were treated with LPS (100 ng/ml) for the time indicated, and phosphorylation levels of p38, ERK, and inhibitor-κB (IκB) were analyzed by immunoblotting. β-Actin immunoblotting was used as a loading control. One representative blot of three independent experiments is shown. C–E, similarly, cells were transfected with pcDNA3 (Vector) or PU.1 and C/EBPα expression vectors for ∼48 h. C, expression of PU.1 and C/EBPα was analyzed by immunoblotting using anti-PU.1 and anti-C/EBPα antibodies, respectively, in B16 cells transfected with vector, PU.1, and/or C/EBPα (left) and in WT B16 and RAW cells (right). Western blots shown are representative of three independent experiments. D, cells were treated with LPS (100 ng/ml) for 6 h, and IL-1β mRNA transcription was analyzed by RT-qPCR. Data are expressed as means ± S.D. (n = 3). E, after transfection, cells were further cultured for 16–18 h, and expression of M-CSFR mRNA was analyzed and compared with that of a housekeeping gene, GAPDH (left). These cells were also cultured in the presence or absence of murine M-CSF (20 ng/ml) and stimulated with LPS (100 ng/ml) for 6 h. IL-1β mRNA transcription was measured by RT-qPCR. Data are expressed as means ± S.D. (n = 2; right).