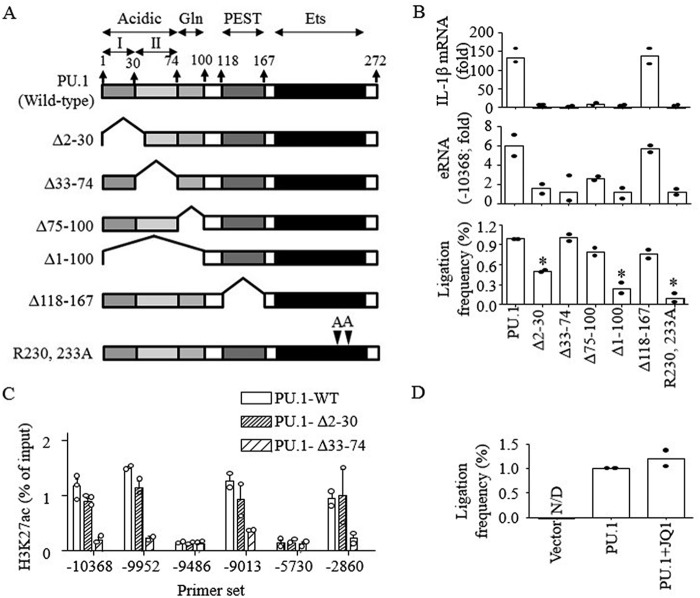
Figure 6.
E2-promoter looping and H3K27ac were mediated by distinct domains of PU.1. A, schematic presentation of PU.1 domains and constructs of PU.1 mutants. B, B16 cells were transfected with PU.1 WT or PU.1 mutant (PU.1Δ2–30, PU.1Δ33–74, PU.1Δ75–100, PU.1Δ1–100, PU.1Δ118–167, and PU.1R230A/R233A) plasmids (1 μg) for 48 h as described under “Experimental procedures,” and cells were stimulated with LPS (100 ng/ml) for 6 h. The expression of IL-1β mRNA (top) and E2 eRNA (middle) was analyzed by RT-qPCR. The proximal localization of E2 and the promoter was analyzed as described under “Experimental procedures” (bottom). Data are expressed as means ± S.D. (n = 2). C, B16 cells were transfected with PU.1 WT or PU.1 mutants, and ChIP assays were performed using anti-H3K27ac as described under “Experimental procedures.” Purified DNAs were then analyzed by qPCR using the primers indicated. Data are presented as percentage of enrichment with the precipitated target sequence compared with input DNA (n ≥ 2). D, proximal localization of DNA fragments of E2 and IL-1β promoter was analyzed in B16 cells transfected with vector or HA-PU.1 plasmids with or without JQ1 (1 μm) treatment. Cumulative values of primer sets 9–13, as shown in Fig. 5A, were plotted. Data are expressed as means ± S.D. (n = 2).