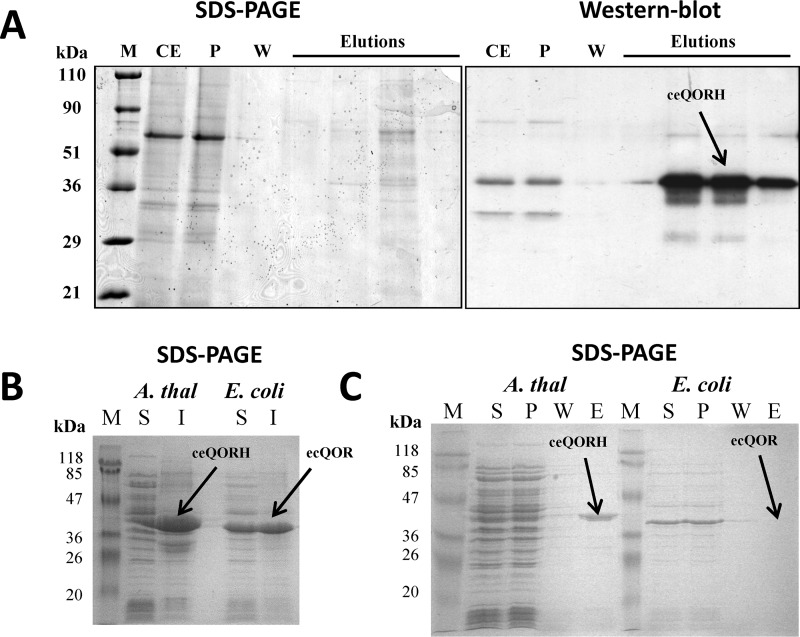
Figure 1.
Interaction of the natural plant ceQORH protein with calmodulin. A, affinity purification of Arabidopsis ceQORH from crude plant cell extracts. Purification was performed on a CaM affinity resin (Stratagene). Lane M, prestained protein molecular weight markers; lane CE, crude solubilized plant proteins diluted in CaM-binding buffer containing 0.1% Nonidet P-40; lane P; unbound proteins; lane W, wash; lane E, four successive elution fractions are presented. B, production of recombinant Arabidopsis ceQORH and E. coli K12 QOR (ecQOR) proteins in E. coli SDS-PAGE analysis of crude bacterial extracts containing Arabidopsis ceQORH or E. coli ecQOR proteins. S, soluble fraction of the crude bacterial proteins; I, insoluble fraction of the crude bacterial proteins (inclusion bodies). C, affinity purification of Arabidopsis ceQORH and E. coli ecQOR produced in bacteria (see B). Purification was performed on a CaM affinity resin (Stratagene). As a control, the bacterial ecQOR protein was also tested. Lane S, soluble bacterial proteins diluted in CaM-binding buffer; lane P, unbound proteins; lane W, wash; lane E, pooled elution fractions. Note that the recombinant Arabidopsis ceQORH protein interacts with the CaM affinity resin (and is thus eluted from the column), whereas this is not the case for the recombinant E. coli ecQOR protein.