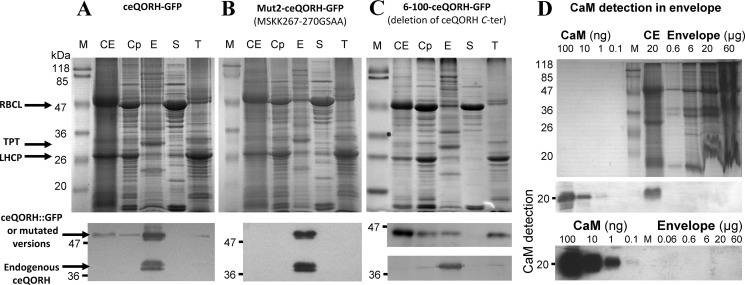
Figure 4.
The ceQORH CaM-binding domain is neither essential for ceQORH targeting to parenchymal cell chloroplasts nor required for specific localization of ceQORH to the plastid envelope. A–C, lack of CaM-binding domain does not affect envelope localization of ceQORH. Cell fractionation was performed on plants stably expressing in A, ceQORH-GFP (ceQORH fused to GFP); in B, Mut2-ceQORH-GFP (ceQORH mutant affected in CaM-binding properties fused to GFP); and in C, 6–100-ceQORH-GFP (ceQORH lacking its 197 C-terminal residues, including the CaM-binding domain, fused to GFP). Lanes M, prestained protein molecular weight markers; lanes CE, crude cell extract; lanes Cp, chloroplast extract; lanes E, envelope; lanes S, stroma; lanes T, thylakoids. Note that to limit artifacts resulting from overexpression of the various ceQORH constructs, transgenic plants were selected for expression levels of recombinant proteins similar to endogenous ceQORH level. Each lane contains 20 μg of proteins. RBCL, large subunit of RuBisCO (stroma marker). LHCP, light-harvesting complex proteins (thylakoid membrane marker). TPT, Triose-P/phosphate translocator (envelope marker). Western blotting analyses were performed using the antibody raised against ceQORH. D, purified chloroplast envelope fractions do not contain detectable levels of CaM. CaM, 100 to 0.1 ng of purified recombinant CaM1 from Arabidopsis. Lane M, prestained protein molecular weight markers. CE, crude cell extract, envelope (0.6–60 μg of envelope proteins). Note that a second CaM detections experiment is shown, using longer exposure time to improve sensitivity of ECL detection.