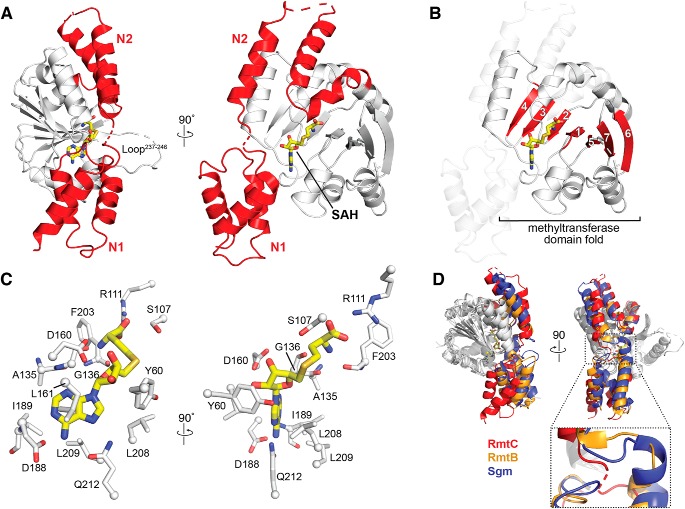
Figure 2.
Structure of the RmtC–SAH complex. A, crystal structure of the RmtC–SAH complex highlighting (red) the extended N-terminal domain characteristic of the aminoglycoside-resistance 16S rRNA (m7G1405) methyltransferases. The N-terminal domain is divided into two subdomains, N1 and N2. The locations of the bound SAH (yellow sticks) and a partially disordered loop (Loop237–246) adjacent to the opening to the SAM-binding pocket are also indicated. B, the same view of the RmtC structure as A (right) but highlighting the seven β-strand core (red) of the C-terminal methyltransferase fold (with N1 and N2 shown as semitransparent cartoon). C, two orthogonal detailed views of the interactions made with SAH in the SAM-binding pocket. D, alignment of RmtC (red) with the structures of RmtB (PDB code 3FRH; orange) and Sgm (PDB code 3LCV; blue), shown in two orthogonal views (top), reveals potential flexibility in the position of the N1 domain relative to the N2/CTD domains via a hinge region between N1 and N2 (zoomed view).