Figure 1.
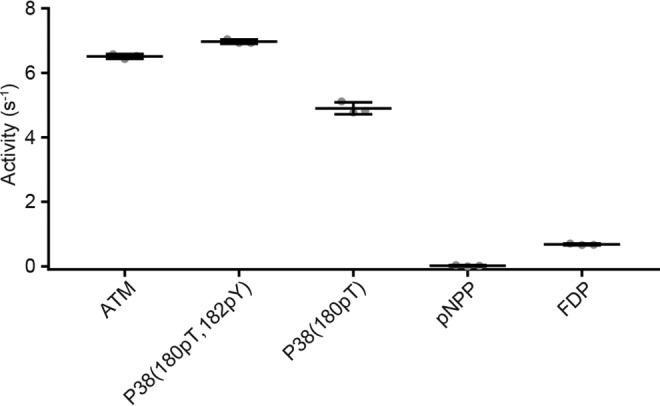
WIP1 is more active against phosphopeptide substrates compared with small molecules commonly used as substrates to measure phosphatase activity. Data show the mean ± S.D. for triplicate activity measurements using a BIOMOL Green assay, where all substrates were tested at 100 μm. The activity of WIP1 toward p-nitrophenyl phosphate (pNPP) is several hundred-fold lower compared with phosphopeptides derived from WIP1 substrates ATM and P38. Similarly, the activity of WIP1 toward fluorescein diphosphate (FDP) is 7–10-fold lower compared with the ATM and P38 phosphopeptide substrates.
