Sir,
PPARs are nuclear receptors that promoted fatty acid metabolism. PPAR α agonists (like fibrates) lower triglyceride and increase HDL levels. PPAR γ agonists (like thiazolidinediones) reduce blood glucose. Dual α/γ PPAR/agonist have the dual promise of reducing both blood glucose and triglyerides and increasing HDL. Saroglitazar is the only dural α/γ PPAR/agonist available in India. The drug also holds the distinction of being an 'Indian' drug and is extensively marketed. However, it wasn't the first drug to act on both PPAR receptors - its closely related molecules were tried in the past and were dropped because of cardiac toxicity. Muraglitazar, developed by Bristol Myers Squibb, was the first drug to reach phase III clinical trials in this class. Nissen et al.,[1] in an RCT concluded that until and unless the safety of muraglitazar was established in a dedicated CVOT, it should not be approved for clinical use in diabetes based on lab end points alone. Tesaglitazar, another drug in the same class, was found to have renal (reduced glomerular filtration rate) and cardiac adverse effects and Astra zeneca, its developer, pulled the plug on further development, Aleglitazar developed by Roche, also met with the same fate. Several other glitazars (navaglitazar, ragaglitazar, farglitazar, chiglitazar) also went to their early graves.[2] Given the failure of the three drugs in this class, the natural questions that arise are, is this increased MACE a class effect or a molecule specific effect? If we consider it a molecule specific effect, how different is saroglitazar (available in Indian market) from muraglitazar/aloglitazar/tezaglitazar on a structural level to believe that it will be different? Finally, do we have a reassuring CVOT to allay the fears, given the chequered history of this molecule's ancestors?
These questions do not have easy answers.
CARDIOVASCULAR SAFETY OF DIABETES DRUGS
The CVOT is a particularly important safety valve, which FDA built into its approval pathway, ensuring that all anti diabetic drugs have to clear the bar of cardiovascular safety, before they reach the market. Given the cracks in post marketing surveillance, such safety precautions are well founded, even if they come at a higher upfront cost. There is currently no consensus on the duration of CVOTs - since most CVOTs are event driven and take large number of patients.
Figure 1 shows the duration of CVOTs in diabetes published so far.
Figure 1.
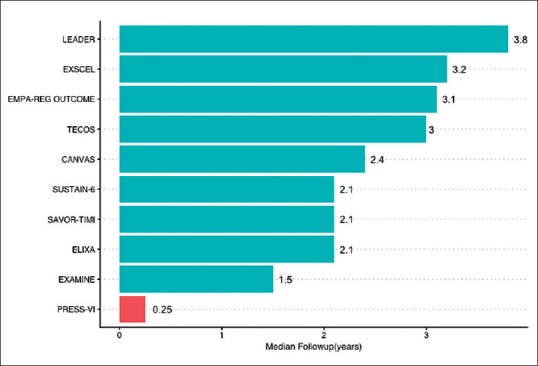
Median Duration of Published CVOTs
Figure 2 shows the sample size of published CVOTS (compared with PRESS -VI for saroglitazar).
Figure 2.
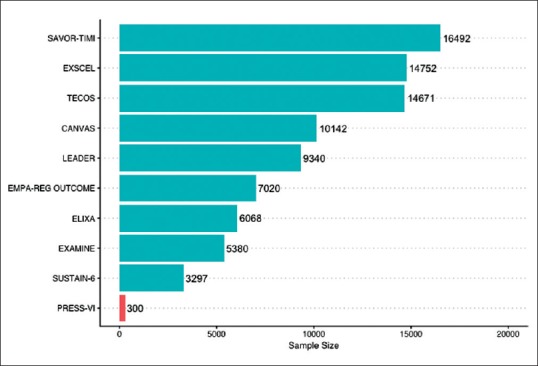
Sample size of published CVOTs
In contrast, the PRESS-VI trial 3 which used saroglitazar has a duration of just 12 weeks with a sample size of 300. The DCGI does not require CVOTs, nor is there is a minimum duration of trial required to ascertain safety. This loophole potentially leaves Indian patients vulnerable to drug-induced adverse effects.
DUAL α/γ PPAR/AGONISTS-MECHANISM OF CARDIAC DYSFUNCTION
The molecular mechanism of cardiac dysfunction induced by glitazars remained obscure. A recent study in mice treated with tezaglitazar throws light on the mechanism.[3] The action of dual PPAR α/γ agonists requires activation of PPAR γ co-activator- 1 (PGC-1). This is a common coactivator for both PPARs and it regulates cardiac fatty acid oxidation, mitochondrial biogenesis and respiration. SIRT1 (sirtuin 1) expression serves as an additional lever of control and regulates PGC-1 expression by acetylation.
A direction reduction in PGC-1, or its deactivation by reduced SIRT1 expression will result in reduction in cardiac mitochondrial number. Glitazars cause competition between PPAR α and γ for PGC-1 and also reduce SIRT1 expression, thereby reducing cardiac mitochondrial abundance. This explains why inspite of being dual agonists, the final effect of glitazars is less than the sum of PPAR α and γ activation.
Interestingly, this reduced SIRT1 expression was ameliorated by resveratrol (found in grapes). Whether the use of resveratrol to reduce cardiac adverse effects of glitazars will work in humans, remains to be seen. Till date, there have been no human studies that have looked at (fibrates + thiazolidinediones) versus glitazars.
CONCLUSION
Following its initial use in diabetes with dyslipidemia (Triglyceride >200), saroglitazar has been studied for use in prediabetes[4] and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NASH),[5,6] Unlike other antidiabetic drugs, saroglitazar has not been tested in high risk patients enrolled in event driven cardiovascular outcome trials. Due to different regulatory requirements in India and its role that straddles two classes of drugs - antidiabetic and lipid lowering - saroglitazar continues to be used in India for various indications. Given the dismal history of its related molecules, its weaker evidence base and the molecular basis of cardiac dysfunction, endocrinologists should exercise caution in using these drugs, especially in those with higher cardiovascular risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–6. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 2.Conlon D. Goodbye glitazars? Br J Diabetes Vasc Dis. 2006;6:135–7. [Google Scholar]
- 3.Kalliora C, Kyriazis ID, Oka S, Lieu MJ, Yue Y, Area-Gomez E, et al. Dual PPARα/γ activation inhibitsSIRT1-PGC1α axis and causes cardiac dysfunction. JCI Insight. 2019;4:e129556. doi: 10.1172/jci.insight.129556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhosle D, Bhosle V, Bobde J, Bhagat A, Shaikh H, Kadam R. Study of saroglitazar in treatment of pre-diabetes with dyslipidemia: STOP-D. J Assoc Physicians India. 2018;66:14–7. [PubMed] [Google Scholar]
- 5.Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084–94. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–90. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]


