Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.1
Keywords: pathology competencies, organ system pathology, gastrointestinal tract, blood supply of gut, ischemic disorders of the gut, vasculitis
Primary Objective
Objective GT2.1: Ischemic Disorders of the Gut: Explain the pathogenesis and clinicopathological features for common disorders of the GI tract that arise from hypoxia or ischemia.
Competency 2: Organ System Pathology; Topic: Gastrointestinal Tract (GT); Learning Goal 2: Anatomy and Blood Supply of the Gut.
Secondary Objectives
Objective CBV3.3: Categories of Vasculitis (Vessel Size): Describe the vasculitides that occur in large, medium, and small vessels.
Competency 2: Organ System Pathology; Topic: Cardiovascular-Blood Vessels (CBV); Learning Goal 3: Vasculitis.
Objective ACD1.2: Necrosis: Define necrosis and compare and contrast the forms of necrosis produced in response to different etiologic agents with respect to their variable clinical and morphologic features.
Competency 1: Disease Mechanisms and Processes; Topic: Adaptation and Cell Death (ACD); Learning Goal 1: Cellular Response to Injury.
Patient Presentation, Part 1
A 50-year-old man presented to the emergency department reporting extreme, unremitting abdominal pain of 2 to 3 hours’ duration. Abdominal pain is accompanied by nausea and nonbloody, nonbilious projectile vomiting. There is no history of diarrhea or constipation. His past medical history includes diabetes, treated with oral hypoglycemic agents, and osteoarthritis, for which he takes over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs). Physical examination reveals a distressed, febrile patient with elevated respiratory rate and pulse and a purpuric rash over the extremities. The abdomen is distended, tense, and tender to palpation, with absent bowel sounds. He received a computed tomography (CT) scan with oral contrast, a complete blood count, and complete metabolic panel.
Diagnostic Findings, Part 1
An abdominal CT scan revealed dilated, thin loops of small intestine. Complete blood count was remarkable for elevated white blood count 18.5 × 109 cells/L (reference 4.3-10.8 × 109 cells/L) and hemoglobin 10 g/dL (reference range: 13.5-16 g/dL). Serum chemistry was remarkable for hemoglobin A1 c of 7.8% (reference range: <6.5%), glucose of 140 mg/dL (reference range random: 79-160 mg/dL), blood urea nitrogen (BUN) of 104 mg/dL (reference range: 5-20 mg/dL), and creatinine of 2.4 mg/dL (reference range: <1.5 mg/dL).
Questions/Discussion Points, Part 1
What Would You Consider in the Differential Diagnosis for This Patient?
The clinical picture is that of acute abdomen, raising a broad differential diagnosis. Important considerations include acute appendicitis or pancreatitis, perforated bowel, such as may be seen in peptic ulcer disease or diverticulitis, intestinal obstruction, ischemia, and ruptured aortic aneurysm.2 In this case, the imaging showed dilated, thin loops of small intestine. Segmental dilatation can occur due to interruption of physiological peristalsis and increased intestinal secretions. These findings are worrisome for bowel ischemia, which could be secondary to veno-occlusive ischemia as well as strangulating obstruction.3
What Are the Major Causes of Ischemic Injury in the Intestine?
Enterocolic ischemia can result from systemic or local compromises in intestinal blood flow. Intestinal ischemia is a frequent consequence of reduced cardiac output, as may result from arrhythmias, heart failure, and shock. Thromboemboli from atherosclerotic disease and coagulopathies and vascular occlusion from mechanical compromise (eg, intussusception, volvulus, strangulated hernias) also reduce blood flow to intestinal segments resulting in ischemia.4 It commonly affects the elderly patients, particularly those with diabetes and hypertensive atherosclerotic cardiovascular disease. Patients with irritable bowel syndrome, chronic obstructive pulmonary disease, and chronic renal failure requiring hemodialysis are at increased risk for ischemic colitis.5,6
Ischemic events may be acute or chronic in nature. Chronic ischemia can affect any intestinal segment and results from etiologies that gradually reduce blood flow to the intestine. Atherosclerotic disease of mesenteric arteries is a common cause of chronic intestinal ischemia and is often referred to as chronic mesenteric ischemia. It produces a clinical syndrome known as intestinal angina, wherein patients experience postprandial abdominal pain due to insufficient blood flow to the small intestine.7 Acute ischemia results from sudden reductions in intestinal blood flow, which may be of mechanical, thromboembolic, or hemodynamic etiology.
Systemic vasculitides, autoimmune diseases, and amyloidosis of various etiologies are also important causes of enterocolic ischemia, as are some infections, particularly those that affect immunosuppressed populations.8 Iatrogenic ischemia may occur postsurgically; intravascular medical devices are often coated with polymers that can dislodge and cause downstream ischemic damage.9 Finally, a growing list of medications, including NSAIDs, is implicated in intestinal ischemia.10 The most common entities and their clinical and histologic features are discussed subsequently.
What Are the Major Features of the Vascular Anatomy of the Tubal Intestine? Which Parts of the Bowel Are More Susceptible to Ischemic Injury?
The vascular supply of the tubal gut is summarized in Table 1. The proximal esophagus receives blood from the inferior thyroidal artery, whereas the mid esophagus derives blood supply from the bronchial and esophageal branches of the thoracic aorta.11 The distal esophagus and stomach are supplied by branches of the celiac artery with a rich anastomotic network between branches that prevents ischemic events, with rare exceptions.11,12 On the other hand, the small intestine and colon are susceptible to ischemic injury. Midgut derivatives, spanning from the ampulla of Vater to splenic flexure of the colon, are supplied by branches of the superior mesenteric artery.13 These vessels also form overlapping arcades within the mesentery, providing collateral vascular supply. The inferior mesenteric artery supplies hindgut derivatives extending from the splenic flexure of the colon to the distal sigmoid colon. This region of relatively low vascularity (“watershed zone”) near the splenic flexure and rectosigmoid junction predisposes it to ischemic injury, often due to nonocclusive causes.14 The cloacal derivatives (distal sigmoid colon, rectum colon, and anus) are supplied by the branches of the internal iliac artery.13
Table 1.
Blood Supply of the Gut.
| Anatomic Site | Major Aortic Branch | Supplying Arteries |
|---|---|---|
| Proximal esophagus | Thyrocervical trunk branch of subclavian artery | Inferior thyroidal artery |
| Mid esophagus | Directly from thoracic aorta | Bronchial and esophageal branches |
| Distal esophagus | Aorta and celiac artery | Ascending branches of the left phrenic and left gastric arteries |
| Gastric cardia | Celiac plexus | Left gastric artery |
| Stomach, greater curvature | Celiac plexus | Splenic and hepatic arteries |
| Stomach, lesser curvature | Celiac plexus | Right gastric artery |
| Proximal duodenum | Superior mesenteric artery | Superior pancreaticoduodenal arteries |
| Distal small intestine | Superior mesenteric artery | Inferior pancreaticoduodenal arteries; jejunal and ileal branches of superior mesenteric artery |
| Ascending and transverse colon | Superior mesenteric artery | Ileocolic, right colic, and middle colic arteries |
| Descending colon to distal sigmoid colon | Inferior mesenteric artery | Marginal artery of Drummond |
| Distal sigmoid colon and rectum | Internal iliac artery | Branches of internal iliac artery |
Patient Presentation, Part 2
While in the emergency department, the patient’s condition acutely declines, and he is taken to the operating room for an exploratory laparotomy.
Diagnostic Findings, Part 2
A segment of the distal ileum is resected. The segment appears dusky red to black with a markedly thin wall and an area of perforation surrounded by fibrinous exudate. The open specimen shows a sharply demarcated area of black mucosa with loss of the usual folds (Figure 1). Histologic sections are shown in Figure 2.
Figure 1.

A segment of small intestine displays a sharply demarcated zone of ischemic necrosis (left), typical of arterial insufficiency (gross image).
Figure 2.
Ischemic intestinal mucosa displays crypt loss and withered “crypts” with cytoplasmic depletion. The lamina propria appears hypereosinophilic due to leakage of serum proteins from injured mucosal blood vessels (A). Other areas contain regenerative crypts with easily identifiable mitotic figures (arrows; B). Hematoxylin and eosin; original magnification: ×400 (A-B).
Questions/Discussion Points, Part 2
What Are the Abnormalities Present in Figure 2? Which Type of Histopathologic Changes Are Depicted in Figure 2A and B?
Figure 2A shows denudation of the small intestinal villi and patchy loss of crypts. The remaining crypts have epithelial cells with attenuated cytoplasm and frequent apoptosis. The lamina propria appears pink due to leakage of serum proteins from rupture injured vessels. Other areas (Figure 2B) display regenerating, immature crypts. This is evidenced by lack of specialized (ie, goblet and Paneth cells) epithelia and frequent mitotic figures. The features are those of acute intestinal ischemia at different stages of development. Arterial insufficiency, from any cause, produces sharply demarcated segmental infarction of the intestine, beginning in the mucosa, which is most vulnerable to hypoxia. Venous insufficiency, on the other hand, is less common and is marked by mural edema and hemorrhage that ultimately progresses to necrosis.
What Are the Main Differential Diagnoses at This Point?
The patient’s medical history includes 2 major risk factors for intestinal ischemia, namely, diabetes and chronic NSAID use. His initial laboratory tests reveal elevated BUN and creatinine and signs of kidney damage that also portend risk for intestinal ischemia. On the other hand, the patient presents acutely and in severe pain. Based on the clinical history, his underlying conditions, including diabetes, arthritis, and renal insufficiency, are chronic in nature and reasonably well controlled. The discordance between his overall state of health and the precipitous and severe nature of this episode raises the possibility of a superimposed vascular compromise.
How Do NSAIDs Cause Intestinal Ischemia? What Other Medications Lead to Intestinal Ischemia?
Nonsteroidal anti-inflammatory drugs induce ischemia by inhibiting cyclooxygenase-1 and cyclooxygenase-2, thereby preventing prostaglandin synthesis. Prostaglandins normally regulate smooth muscle contraction in blood vessel walls and prevent clot formation. The proximal duodenum, ileum, and right colon are most susceptible to NSAID-induced ischemia. Ischemia is usually localized, but rare, life-threatening bleeding may result from NSAID use.15 Hormone therapies, particularly oral contraceptive pills, induce ischemia by inducing resistance to intrinsic antithrombotic mechanisms. Cocaine and other recreational drugs promote vasospasm and induce ischemia. Opioid and antipsychotic medications cause constipation and bowel distention, exerting direct pressure on mural blood vessels.10
What Histologic Changes Are Present in the Deeper Vessels of the Intestinal Wall Shown in Figure 3A and 3B?
Figure 3.
The medium-sized arteries show mixed inflammation in their walls and areas of fibrinoid necrosis (arrow; A). More advanced vascular lesions feature intimal proliferation with near-occlusion of the vessel lumen (B). Hematoxylin and eosin; original magnification: ×400 (A-B).
Deeper in the intestine wall, the medium-sized arteries contain mixed inflammation and fibrinoid necrosis (Figure 3A). Other vessels have markedly thickened intima with slit-like residual lumina (Figure 3B).
How Do These Findings Change the Differential Diagnosis?
These pathologic changes in the mural blood vessels raise the possibility of a systemic vasculitis as the cause of ischemia. The patient has several other risk factors for ischemia, as discussed, but these etiologies are not associated with vessel inflammation, necrosis, or remodeling.
Diagnostic Findings, Part 3
Additional history reveals that the patient had noticed tingling and numbness in his fingers over the past several months. He does not have any history of autoimmune disorders, chronic sinusitis, asthma, or recent upper respiratory infection. He also has no history of malignancy or other serious illness. Serologic tests, including for perinuclear or cytoplasmic antineutrophil cytoplasmic antibodies (p-ANCA and c-ANCA, respectively), were ordered. On laboratory testing, he is negative for p-ANCA and c-ANCA. In the absence of immune-mediated causes for his symptoms, possible infectious causes of vasculitis should be considered, particularly in patient presenting with fever and leukocytosis. This disease workup revealed positive hepatitis B surface antigen and immunoglobulin G anti-hepatitis B core, indicating chronic hepatitis B infection.
Questions/Discussion Points, Part 3
What Are the Clinical Manifestations and Histopathologic Findings of Vasculitides That Affect the Gastrointestinal Tract?
Many systemic vasculitides have gastrointestinal tract manifestations (Table 2). These diseases display overlapping features and require correlation with serologic findings and other clinical data for accurate classification. In general, large vessel vasculitides do not involve the gastrointestinal tract. Medium vessel vasculitides that may have intestinal manifestations include polyarteritis nodosa (PAN), granulomatosis with polyangiitis (formerly called, Wegener granulomatosis), and eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss syndrome).16 Polyarteritis nodosa is a necrotizing systemic vasculitis that frequently affects the skin, kidneys, peripheral nervous system, and gastrointestinal tract. It may be idiopathic but is frequently associated with chronic hepatitis B infection.17 Patients with granulomatosis with polyangiitis are commonly positive for c-ANCA.18 Granulomatosis with polyangiitis manifests with cough, hemoptysis, pleuritis, and rapidly progressive glomerulonephritis.19 Vasculitis is characterized by vessel wall necrosis and extravascular granulomas. Eosinophilic granulomatosis with polyangiitis is often marked by positive p-ANCA. It produces chronic allergic rhinitis, nasal polyposis, and asthma.20 The vasculitis features eosinophil-rich infiltrates and occasional granulomas. Rheumatoid arthritis (RA) is a systemic autoimmune disorder that may cause inflammation of medium-sized vessels of the gastrointestinal tract.
Table 2.
Clinical and Pathologist Features of Systemic Vasculitides Affecting the Gastrointestinal Tract.
| Disorder | Key Manifestations | Inflammatory Pattern |
|---|---|---|
| Polyarteritis nodosa | Renal failure, neuropathies, orchitis Gastrointestinal involvement: 30%-50% |
Fibrinoid necrosis of medium-sized arteries Mixed inflammatory infiltrate |
| Granulomatosis with polyangiitis (Wegener) | Cough, hemoptysis, rapidly progressive glomerulonephritis,
c-ANCA Gastrointestinal involvement: 5%-10% |
Necrotizing vasculitis with extravascular granulomas |
| Churg-Strauss syndrome | Asthma, sinusitis, neuropathy, peripheral eosinophilia,
p-ANCA Gastrointestinal involvement: 30%-60% |
Necrotizing vasculitis with dense, eosinophil-rich inflammation and extravascular granulomas |
| Microscopic polyangiitis | Fever, arthralgias, weight loss, rapidly progressive glomerulonephritis,
p-ANCA Gastrointestinal involvement: 30%-50% |
Necrotizing vasculitis of arterioles, capillaries, venules Sparing of small arteries and veins |
| Henoch-Schonlein purpura | Most common vasculitis in children Sequela of streptococcal pharyngitis in children and young adults Nonblanching purpura, arthralgias, nephritis, diarrhea Gastrointestinal involvement: 60%-70% |
Lamina propria hemorrhage and fibrin deposits, neutrophilic enteritis or
colitis with erosions Leukocytoclastic vasculitis: fibrinoid vessel wall necrosis with neutrophil-rich infiltrates in capillaries, arterioles, and venules seen in minority of cases IgA deposits in vessel walls on immunofluorescence |
| Systemic lupus erythematosus | Multi-organ involvement results in variable
presentation Gastrointestinal involvement: 15% |
Fibrinoid necrosis of venules and arterioles Immune complex deposits on immunofluorescence |
| Rheumatoid arthritis | Chronic inflammatory disorder mainly affecting joints Gastrointestinal involvement: 25% |
Necrotizing vasculitis of small- to medium-sized arteries |
Abbreviations: c-ANCA, cytoplasmic antineutrophil cytoplasmic antibody; IgA, immunoglobulin A; p-ANCA, perinuclear antineutrophil cytoplasmic antibody.
Small vessel vasculitides that involve the gastrointestinal tract include microscopic polyangiitis and leukocytoclastic vasculitis (Henoch-Schonlein purpura).21 Microscopic polyangiitis is a p-ANCA-associated disorder that causes arthralgias and rapidly progressive glomerulonephritis in addition to intestinal ischemia.22 Leukocytoclastic vasculitis usually affects children and follows upper respiratory infection, particularly with group A hemolytic Streptococcus spp. Patients experience a purpuric skin rash, arthralgias, nephritis, and diarrhea. In addition, systemic lupus erythematosus (SLE) causes small vessel inflammation in the gastrointestinal tract.16
What Is the Most Likely Diagnosis?
Absence of ANCAs and respiratory symptoms eliminates the possibilities of granulomatosis with polyangiitis and EGPA. The patient reports no other systemic symptoms prior to this episode, making rheumatologic disorders, such as SLE and RA, unlikely. The absence of recent infection and older age make Henoch-Schonlein purpura unlikely. On the other hand, neurologic symptoms, serologic evidence of kidney failure, and hepatitis B infection point to PAN. This diagnosis is also supported by the presence of necrotizing vasculitis of medium-sized vessels.
Which Other Infectious and Inflammatory Disorders May Manifest as Enterocolic Ischemia?
Cytomegalovirus
Common patterns of cytomegalovirus (CMV)-associated enterocolitis range from mild active inflammatory infiltrate with patchy cryptitis and crypt abscesses to ulcers and mucosal and mural necrosis. Cytomegalovirus produces both nuclear and cytoplasmic inclusions in endothelial cells, stromal cells, macrophages, and, rarely, in glandular epithelial cells. Nuclear inclusions are amphophilic and surrounded by a clear zone, resulting in an “owl’s eye” appearance, whereas cytoplasmic inclusions are granular and eosinophilic (Figure 4).23 Cytomegalovirus vasculitis is an uncommon cause of segmental ischemia. Cytomegalovirus vasculitis features neutrophil-rich vessel wall inflammation, characteristic inclusions in endothelial cells, and luminal narrowing.24
Figure 4.

Cytomegalovirus-associated vasculitis features mixed inflammation in and around vessel walls. Endothelial cells bearing “owl’s eye” inclusions (arrows) are the key to the diagnosis. Hematoxylin and eosin; original magnification: ×400.
Enterohemorrhagic Escherichia coli
Enterohemorrhagic Escherichia coli is a group of Shiga toxin-producing bacteria, of which E coli O157:H7 is the most common strain. Enterohemorrhagic E coli shows a predilection for the ascending and transverse colon and produces mucosal hemorrhage, longitudinal ulcers, and, sometimes, pseudomembranes. The colonic mucosa shows acute inflammation and superficial hemorrhagic necrosis with sparing of the deeper compartment (Figure 5). Ischemic injury, evidenced by crypt “withering” and lamina propria hyalinization, is prominent. Necrosis of small- and medium-sized blood vessels with intraluminal fibrin thrombi may also be seen.25,26
Figure 5.

Enterohemorrhagic Escherichia coli infection produces ischemic colitis with crypt loss and lamina propria hyalinization. Prominent mucosal neutrophils are also characteristic of the infection. Hematoxylin and eosin; original magnification: ×400.
Angioinvasive fungi
The most common angioinvasive fungi include Aspergillus and Mucor spp; disseminated infection is typically limited to immunosuppressed populations.27,28 Aerosolized Aspergillus spores, most commonly Aspergillus niger, colonize the upper respiratory tract and may disseminate to the gastrointestinal tract in immunocompromised hosts.27 The bowel wall typically shows ischemic infarction with hyphae emanating from thrombosed blood vessels in radial arrays. Aspergillus hyphae are septate, have parallel walls, and branch at acute angles. Fungi in the Mucorales order have pauciseptate hyphae with irregular walls and “ribbon-like” folds that branch at random angles (Figure 6). They appear optically clear when sectioned transversely. The mortality of gastrointestinal infection with these organisms can reach 50%.23
Figure 6.
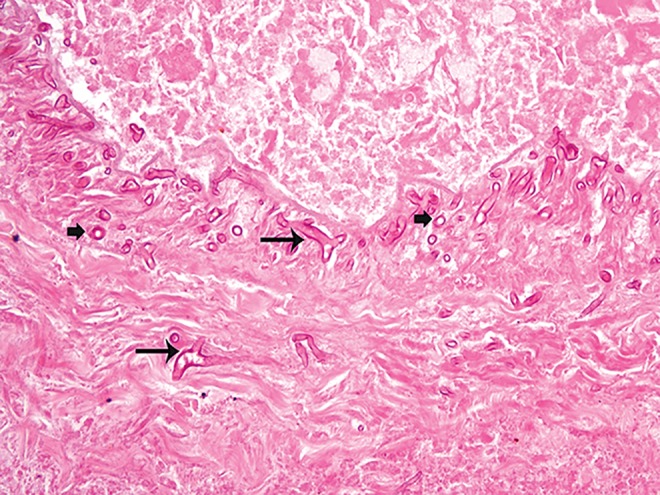
Mucorales organisms migrate through a necrotic blood vessel wall in this infarcted intestinal segment. They have broad, pauci-septate hyphae that branch at random angles (shown by large arrows) and appear optically clear when sectioned transversely (shown by small block arrows). Hematoxylin and eosin; original magnification: ×400.
Amyloidosis
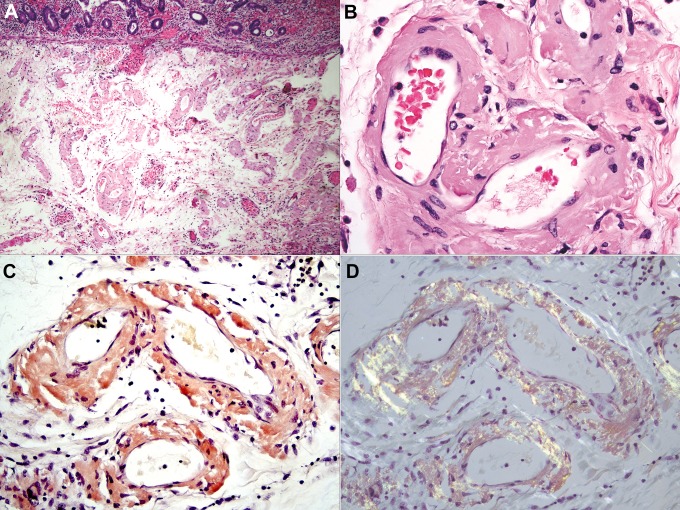
Systemic amyloidosis results from plasma cell neoplasms, chronic inflammatory conditions, kidney failure, and hereditary defects in protein folding.29 Amyloidoses that affect the gastrointestinal tract and their parent proteins are summarized in Table 3.30 Amyloid may deposit in any layer of the intestinal wall, and deposits are usually asymptomatic. Substantial deposition in tissue in and around blood vessels in the colon and small intestine leads to ischemia (Figure 7A).31 All forms are morphologically identical and appear as waxy, eosinophilic extracellular material with artifactual “cracks” (Figure 7B). The material stains positive with Congo red stains (Figure 7C) and displays “apple red birefringence” when polarized (Figure 7D). Subtyping of amyloid by standard histochemical technique is generally not possible, but mass spectrometry techniques may be helpful.32
Table 3.
Systemic Amyloidoses Affecting the Gastrointestinal Tract.
| Type of Amyloid | Source | Clinical Condition |
|---|---|---|
| AL | Kappa or lambda immunoglobulin light chains | Plasma cell myeloma or lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia) |
| AH | Immunoglobulin heavy chains | Plasma cell dyscrasias |
| AA | Serum amyloid A: acute phase reactant | Chronic inflammatory diseases: inflammatory bowel disease, rheumatoid arthritis, familial Mediterranean fever |
| ATTR | Tissue transthyretin | Part of normal aging |
| ATTR | Hereditary amyloidosis | |
| Aβ2M | β2-Microglobulin | Ineffective renal clearance in dialysis patients |
Figure 7.
Ischemic colonic mucosa overlies markedly thickened submucosal blood vessels (A). The vessels are expanded by amyloid deposits, which are deeply eosinophilic in hematoxylin and eosin (H&E) stains and show a characteristic “cracking” artifact (B). The deposits stain red with Congo red stains (C) and show “apple green” birefringence upon polarization (D). H&E (A and B); Congo red (C and D); original magnifications: ×40 (A), ×400 (B-D).
What Therapies Are Available for Intestinal Ischemia and What Is the Prognosis?
Most patients with intestinal ischemia are managed conservatively with supportive care and broad-spectrum antibiotics, although patients with extensive or recurrent disease require surgical resection.4 Outcomes are heavily influenced by extent of intestinal involvement and hemodynamic instability.33 Treatment is aimed at the underlying cause for patients with systemic disorders. Systemic inflammatory disorders generally require immunosuppressive therapy, whereas infections are managed with appropriate antimicrobial agents. Amyloidosis from plasma cell dyscrasias requires chemotherapy, but treatment options are limited for patients with hereditary forms of the disease.34,35
Teaching Points
Intestinal ischemia can result from localized or systemic vascular compromise and preferentially effects intestinal segments with less collateral circulation. The colon is most commonly affected due to the presence of watershed areas like splenic flexure and rectosigmoid area.
Intestinal ischemia is a potential complication of commonly used over-the-counter and prescription medications including NSAIDs and oral contraceptive pills.
Systemic vasculitides (granulomatosis with polyangiitis, EGPA, PAN, microscopic polyangiitis, leukocytoclastic vasculitis) and autoimmune diseases (SLE, RA) can present with intestinal ischemia.
Infections that can cause ischemia (CMV, angioinvasive fungi) are important to consider, particularly in immunocompromised patients.
Integration of subtle histologic clues and clinical data may elucidate the cause of intestinal ischemia and guide clinical management in a substantial proportion of cases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The article processing fee for this article was supported by an Open Access Award given by the Society of ‘67, which supports the mission of the Association of Pathology Chairs to produce the next generation of outstanding investigators and educational scholars in the field of pathology. This award helps to promote the publication of high-quality original scholarship in Academic Pathology by authors at an early stage of academic development.
ORCID iD: Priyanka Patil  https://orcid.org/0000-0001-6213-044X
https://orcid.org/0000-0001-6213-044X
References
- 1. Knollmann-Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017:4 doi:10.1177/2374289517715040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattson B, Dulaimy K. The 4 quadrants: acute pathology in the abdomen and current imaging guidelines. Semin Ultrasound CT MR. 2017;38:414–423. [DOI] [PubMed] [Google Scholar]
- 3. Furukawa A, Kanasaki S, Kono N, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192:408–416. [DOI] [PubMed] [Google Scholar]
- 4. Feuerstadt P, Brandt LJ. Update on colon ischemia: recent insights and advances. Curr Gastroenterol Rep. 2015;17:45. [DOI] [PubMed] [Google Scholar]
- 5. Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004;19:729–738. [DOI] [PubMed] [Google Scholar]
- 6. Flobert C, Cellier C, Berger A, et al. Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am J Gastroenterol. 2000;95:195–198. [DOI] [PubMed] [Google Scholar]
- 7. Jaster A, Choudhery S, Ahn R, et al. Anatomic and radiologic review of chronic mesenteric ischemia and its treatment. Clin Imaging. 2016;40:961–969. [DOI] [PubMed] [Google Scholar]
- 8. Uberti G, Goldblum JR, Allende DS. Ischemic enterocolitis and its differential diagnosis. Semin Diagn Pathol. 2014;31:152–164. [DOI] [PubMed] [Google Scholar]
- 9. Chavez JA, Chen W, Frankel WL, Arnold CA. Hydrophilic polymer-associated ischemic enterocolitis. Am J Surg Pathol. 2017;41:271–276. [DOI] [PubMed] [Google Scholar]
- 10. Vodusek Z, Feuerstadt P, Brandt LJ. Review article: the pharmacological causes of colon ischaemia. Aliment Pharmacol Ther. 2019;49:51–63. [DOI] [PubMed] [Google Scholar]
- 11. Aida J, Vieth M, Ell C, et al. Palisade vessels as a new histologic marker of esophageal origin in ER specimens from columnar-lined esophagus. Am J Surg Pathol. 2011;35:1140–1145. [DOI] [PubMed] [Google Scholar]
- 12. Piasecki C. Blood supply to the human gastroduodenal mucosa with special reference to the ulcer-bearing areas. J Anat. 1974;118:295–335. [PMC free article] [PubMed] [Google Scholar]
- 13. Sise MJ. Acute mesenteric ischemia. Surg Clin North Am. 2014;94:165–181. [DOI] [PubMed] [Google Scholar]
- 14. Wilcox MG, Howard TJ, Plaskon LA, Unthank JL, Madura JA. Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci. 1995;40:709–716. [DOI] [PubMed] [Google Scholar]
- 15. Panarelli NC. Drug-induced injury in the gastrointestinal tract. Semin Diagn Pathol. 2014;31:165–175. [DOI] [PubMed] [Google Scholar]
- 16. Chetty R, Serra S. A pragmatic approach to vasculitis in the gastrointestinal tract. J Clin Pathol. 2017;70:470–475. [DOI] [PubMed] [Google Scholar]
- 17. Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017;13:381–386. [DOI] [PubMed] [Google Scholar]
- 18. Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA). Autoimmunity. 2005;38:93–103. [DOI] [PubMed] [Google Scholar]
- 19. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis). J Autoimmun. 2014;48-49:94–98. [DOI] [PubMed] [Google Scholar]
- 20. Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68:261–273. [DOI] [PubMed] [Google Scholar]
- 21. Louie CY, Gomez AJ, Sibley RK, Bass D, Longacre TA. Histologic features of gastrointestinal tract biopsies in iga vasculitis (Henoch-Schönlein Purpura). Am J Surg Pathol. 2018;42:529–533. [DOI] [PubMed] [Google Scholar]
- 22. Greco A, De Virgilio A, Rizzo MI, et al. Microscopic polyangiitis: advances in diagnostic and therapeutic approaches. Autoimmun Rev. 2015;14:837–844. [DOI] [PubMed] [Google Scholar]
- 23. Panarelli NC, Yantiss RK. Inflammatory and infectious manifestations of immunodeficiency in the gastrointestinal tract. Mod Pathol. 2018;31:844–861. [DOI] [PubMed] [Google Scholar]
- 24. Chen YM, Hung YP, Huang CF, et al. Cytomegalovirus disease in nonimmunocompromised, human immunodeficiency virus-negative adults with chronic kidney disease. J Microbiol Immunol Infect. 2014;47:345–349. [DOI] [PubMed] [Google Scholar]
- 25. Kelly J, Oryshak A, Wenetsek M, Grabiec J, Handy S. The colonic pathology of Escherichia coli O157: H7 infection. Am J Surg Pathol. 1990;14:87–92. [DOI] [PubMed] [Google Scholar]
- 26. Griffin PM, Olmstead LC, Petras RE. Escherichia coli O157: H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology. 1990;99:142–149. [DOI] [PubMed] [Google Scholar]
- 27. Kazan E, Maertens J, Herbrecht R, et al. A retrospective series of gut aspergillosis in haematology patients. Clin Microbiol Infect. 2011;17:588–594. [DOI] [PubMed] [Google Scholar]
- 28. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54:S23–34. [DOI] [PubMed] [Google Scholar]
- 29. Iida T, Yamano H, Nakase H. Systemic amyloidosis with gastrointestinal involvement: Diagnosis from endoscopic and histological views. J Gastroenterol Hepatol. 2018;33:583–590. [DOI] [PubMed] [Google Scholar]
- 30. Sipe JD, Benson MD, Buxbaum JN, et al. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21:221–224. [DOI] [PubMed] [Google Scholar]
- 31. Freudenthaler S, Hegenbart U, Schonland S, Behrens HM, Kruger S, Rocken C. Amyloid in biopsies of the gastrointestinal tract—a retrospective observational study on 542 patients. Virchows Arch. 2016;468:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winter M, Tholey A, Kristen A, Rocken C. MALDI mass spectrometry imaging: a novel tool for the identification and classification of amyloidosis. Proteomics. 2017;17 doi:10.1002/pmic.201700236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ, American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol. 2015;110:18–44. [DOI] [PubMed] [Google Scholar]
- 34. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–2654. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki K. Diagnosis and treatment of multiple myeloma and AL amyloidosis with focus on improvement of renal lesion. Clin Exp Nephrol. 2012;16:659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]