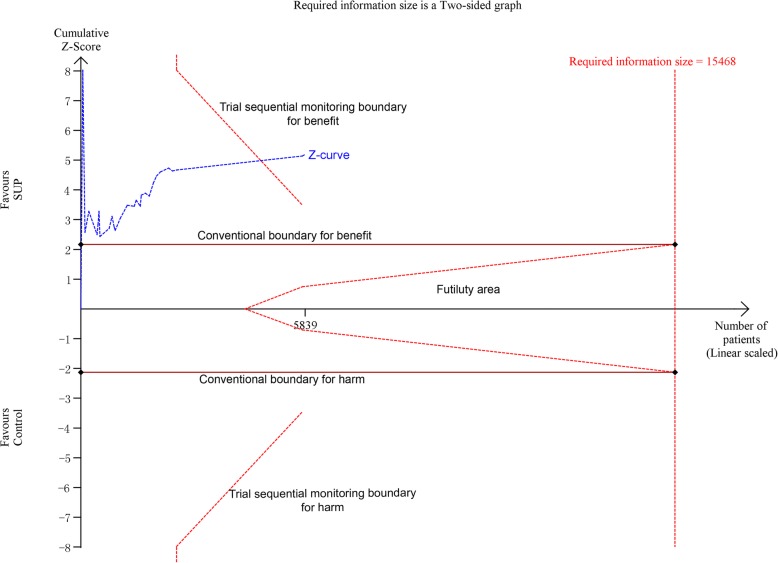
Fig. 4.
Trial sequential analysis for the overt GI bleeding in all included trials. Trial sequential analysis using random-effects model with an adjusted type I error rate of 3.3%, power of 80%, D2 of 62%, for an relative risk reduction of 20% in control event proportion of 12.1%. The Z-curve cross the trial sequential monitoring boundary for benefit, but do not reach the required information size of 15,468 participants. The TSA-adjusted 95% CI for an RR of 0.48 is 0.31 to 0.75. SUP stress ulcer prophylaxis; GI gastrointestinal; TSA trial sequential analysis