Abstract
Purpose:
This study aimed to investigate whether 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU), a soluble epoxide hydrolase inhibitor with anti-inflammatory effects, could alleviate spontaneous recurrent seizures (SRS) and epilepsy-associated depressive behaviours in the lithium chloride (LiCl)-pilocarpine-induced post-status epilepticus (SE) rat model.
Methods:
The rats were intraperitoneally (IP) injected with LiCl (127 mg/kg) and pilocarpine (40 mg/kg) to induce SE. A video surveillance system was used to monitor SRS in the post-SE model for 6 weeks (from the onset of the 2nd week to the end of the 7th week after SE induction). TPPU (0.1 mg/kg/d) was intragastrically given for 4 weeks from the 21st day after SE induction in the SRS + 0.1 TPPU group. The SRS + PEG 400 group was given the vehicle (40% polyethylene glycol 400) instead, and the control group was given LiCl and PEG 400 but not pilocarpine. The sucrose preference test (SPT) and forced swim test (FST) were conducted to evaluate the depression-like behaviours of rats. Immunofluorescent staining, enzyme-linked immunosorbent assay, and western blot analysis were performed to measure astrocytic and microglial gliosis, neuronal loss, and levels of soluble epoxide hydrolase (sEH), cytokines [tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6], and cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB).
Results:
The frequency of SRS was significantly decreased at 6 weeks and 7 weeks after SE induction in the 0.1TPP U group compared with the SRS + PEG 400 group. The immobility time (IMT) evaluated by FST was significantly decreased, whereas the climbing time (CMT) was increased, and the sucrose preference rate (SPR) evaluated by SPT was in an increasing trend. The levels of sEH, TNF-α, IL-1β, and IL-6 in the hippocampus (Hip) and prefrontal cortex (PFC) were all significantly increased in the SRS + PEG 400 group compared with the control group; neuronal loss, astrogliosis, and microglial activation were also observed. The astrocytic and microglial activation and levels of the pro-inflammatory cytokines in the Hip and PFC were significantly attenuated in the TPPU group compared with the SRS + PEG 400 group; moreover, neuronal loss and the decreased CREB expression were significantly alleviated as well.
Conclusion:
TPPU treatment after SE attenuates SRS and epilepsy-associated depressive behaviours in the LiCl-pilocarpine induced post-SE rat model, and it also exerts anti-inflammatory effects in the brain. Our findings suggest a new therapeutic approach for epilepsy and its comorbidities, especially depression.
Keywords: Epilepsy, Depression, Comorbidity, Soluble epoxide hydrolase, Inflammation
Introduction
Epilepsy is a chronic brain disease, which not only has the clinical feature of recurrent seizures but also has cognitive and psychological comorbidities, especially when patients have active refractory seizures1. There is a strong bidirectional relationship between epilepsy and depression2. Certain common neurobiological mechanisms are found to contribute to the comorbidity of epilepsy and depression3. Selective serotonin reuptake inhibitors (SSRIs) are the antidepressants most recommended to alleviate depressive symptoms in patients with epilepsy, but they have a high risk of exacerbating seizures, especially when using in overdose 4, 5. There currently is no effective therapeutic method for simultaneously treating the epilepsy and depression. Emerging evidence indicates that neuroinflammation plays an important role in epileptogenesis in both humans with epilepsy and animal models of epilepsy6, 7. Over-production of inflammatory factors such as interleukin (IL)-1β, IL-6, tumour necrosis factor alpha (TNF-α), and prostaglandin E2 (PGE2) contributes to the progression of seizures8, 9. Activation of inflammatory mediators such as cyclooxygenase (COX)-2 was also found to induce neuronal damage and facilitate seizures10, 11. Glial cell activation may potentiate seizures by increasing pro-inflammatory cytokines and inducing the dysfunction of neuron-glial communication12, 13. Moreover, Mazarati et al. found that the hippocampal IL-1β was a contributing factor for depressive behaviours in a pilocarpine-induced status epilepticus (SE) model, and blockade of hippocampal IL-1 receptor (IL-1R) exerted an anti-depressant effect in the post-SE model14, indicating that an inflammatory mechanism may be closely involved in epilepsy-associated depression as well. Treatments targeting neuroinflammation might present a novel therapeutic strategy for patients with epilepsy and neurobehavioural comorbidities15.
In recent years, the arachidonic acid (AA) metabolic pathway and its roles in inflammation have been widely studied. AA is an abundant unsaturated fatty acid stored in membrane phospholipids. Free AA is metabolised into active intermediate substrates by COX, lipoxygenase (LOX), and cytochrome P450 (CYP450) epoxygenases, of which CYP450 epoxygenases metabolise AA into different types of epoxyeicosatrienoid acids (EETs). EETs have a variety of beneficial functions including anti-inflammatory effects, vasodilation, and even exerting neuroprotective effects16. However, EETs are easily hydrolysed by soluble epoxide hydrolase (sEH) to form the corresponding diols with reduced biological activity17. CYP450 epoxygenases and sEH are widely expressed in neurons, astrocytes, and microvascular endothelial cells in the cortex and hippocampus18. Studies showed that sEH gene knockout or inhibiting the activity of sEH could enhance the beneficial effects of EETs, and that inhibitors of sEH (sEHI) have potent anti-inflammatory effects19.
It has been demonstrated that the level of sEH was significantly elevated in the temporal cortex and hippocampal complexes of patients with temporal lobe epilepsy (TLE)20. In a mouse model of acute tetramethylenedisulfotetramine intoxication, post-exposure administration of sEHI in combination with diazepam effectively prevented progression to tonic seizures and animal death probably mediated by the potent anti-inflammatory effects of sEHI21. Another study found that the expression of sEH protein was higher in the brain of chronically stressed mice and post-mortem brain samples of patients with psychiatric diseases than their controls, and it showed that pre-treatment with sEHI prevented depression-like behaviours in an inflammation-induced depression model, indicating that sEH also plays a key role in the pathophysiology of depression22. In this study, we aimed to investigate the effects of treatment with 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) 21 days after SE, a type of sEHI that can cross the blood-brain barrier 23, on seizures and the epilepsy-associated depressive behaviours in the lithium chloride (LiCl)-pilocarpine-induced post-SE rat model. Moreover, markers for inflammation and cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB) in the neuronal survival pathway were measured to explore the underlying mechanism of TPPU.
Methods
1. Animals
Male adult Sprague-Dawley rats, aged 6 to 8 weeks and weighing 200 to 250 g (supplied by Shanghai Charles River Laboratory), were used in this study. The rats were housed four per cage at ambient temperature 22°C to 25°C and under a 12-hour day-night cycle with free access to food and water. The experiment was done in accordance with the guidelines of the National Institutes of Health. The Committee of Animal Care and Use in Zhongshan Hospital of Fudan University (Shanghai, China) approved this study. Efforts were made to minimise animal suffering and to reduce the number of animals used.
2. Establishment of the LiCl-pilocarpine-induced post-SE rat model
The LiCl-pilocarpine-induced post-SE rat model was established as described previously24. Briefly, rats received intraperitoneal (IP) injections of LiCl (127 mg/kg, dissolved in water, Sigma, St. Louis, MO, USA). After 24 hours, scopolamine methyl bromide (1 mg/kg, Sigma-Aldrich, USA) was given IP to the rats to reduce peripheral muscarinic effects. Then, 30 minutes later the muscarinic agonist pilocarpine (40 mg/kg, Sigma-Aldrich, USA) was IP injected to induce SE. Seizures started 10 to 30 minutes after the pilocarpine injection. A modified Racine scale was used to evaluate seizure severity25. The criterion of SE in this study was that recurrent seizures greater than or equal to Racine stage 4 lasted for 30min. At 30 minutes after seizure onset, only rats arriving SE were treated with diazepam (10 mg/kg, Tianjin, China) to terminate seizures, otherwise they were excluded from the experiment.
Pilocarpine administration to rats results in SE, and after a latency period the SRS occurs. According to a previous research, the latency period is about 7.2±3.6d after SE 26, so the rats that survived the first week after SE were monitored with a video surveillance system (a CCD camera, JVC, Japan) to observe their SRS in our study. Every 3–4 rats were kept in a transparent cage, marked with picric acid on their ears or legs to make a distinction with each other. The monitoring period lasted 6 weeks (from the onset of the 2nd week to the end of the 7th week after SE induction). Qinglan Chen who was blinded to the grouping and drug administration visually inspected the video-recorded data with 6h/d in the daytime for 6 weeks; only SRS reaching a Racine stage 3 to 5 (rearing and/or rearing and falling) were included for further analysis.
3. Treatment groups
TPPU was synthesised in the laboratory of Prof. Bruce D. Hammock at the University of California, Davis, as previously described27. TPPU was dissolved in a saline solution containing 40% polyethylene glycol 400 (PEG 400), and the volumes of 1–1.5ml TPPU (0.1 mg/kg/d, abbreviated as 0.1 TPPU) were administered by gastric gavage at 8am every morning, as the half-life of elimination phase of TPPU in murine models could be over 24h23.
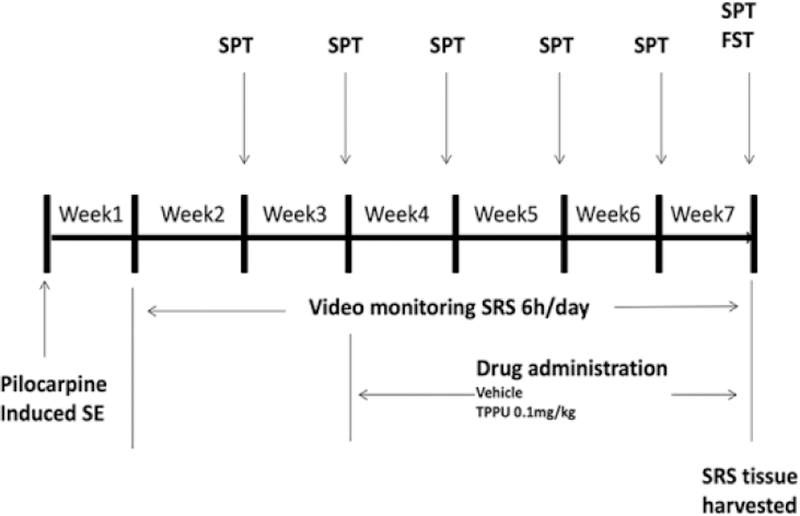
Rats in the LiCl-pilocarpine induced post-SE model were divided into two groups as follows: 1) the SRS + 0.1 TPPU group was given 0.1 mg/kg TPPU for 4 weeks from the 21st day after SE induction; 2) the SRS + PEG 400 group was given the vehicle (PEG 400) instead of TPPU. The control group received LiCl and PEG 400, but not pilocarpine. The rats were observed for SRS from the 8th day after SE induction, and then the brain tissues were harvested after performing the FST and SPT at 7w after SE induction (see Fig. 1).
Fig. 1.
Schematic diagram showing the timeline for drug administration and behavioural testing.
4. Depression-like behavioural tests
Sucrose preference test (SPT)
The procedure of this test is consistent with the previous study24. This test is for the depressive behaviour of anhedonia based on the innate preference of rodents toward sweets28. The rats were deprived of water for 24 hours before the test. On the day of test, two identical bottles, one of regular water and one with water containing 1% sucrose, were put on every cage. At 30 minutes after the onset of the test, the locations of the two bottles were exchanged. The test lasted for 1 hour, and then liquid intakes were calculated, as follows: sucrose preference rate (SPR) = sucrose consumption/(sucrose consumption + water consumption) × 100%. A low SPR was indicative of the state of anhedonia. The SPT was performed once every week after 2 weeks from SE induction (at the same day of every week from 9am to 10am in the morning) in the control, SRS + PEG 400, and SRS + 0.1 TPPU groups (see Fig. 1).
Forced Swim Test (FST)
The FST was performed in the daytime when 6h-monitoring had been completed. The aim of FST is to test the state of despair29. The rat is put into a transparent tank (60 cm height × 30 cm diameter) filled with water 22°C to 25°C in temperature. The swimming behaviours of rats were observed for 5 minutes. The three types of swimming behaviours, including immobile behaviour, climbing behaviour, and swimming behavior, recorded by videotapes were manually analysed according to our previous procedure 24 The longer immobility time (IMT) represents the behaviour of despair, whereas the longer climbing time (CMT) indicates the active behaviour of rats. The FST was performed at 7 weeks after SE induction in the control, SRS + PEG 400, and SRS + 0.1 TPPU groups (see Fig. 1).
To avoid the immediate influence of seizures on the outcome of behavioural assay, the SPT and FST were performed after verifying that no seizures had developed for at least 6 hours before the tests.
5. Immunofluorescent staining
At the end of the behavioural observation, the rats were deeply anesthetized with 10% chloral hydrate (3 mL/kg, IP) and perfused trans-cardinally with 4°C saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS) (10 mM, pH 7.4). After that, rats were decapitated and their brains were removed and stored in 4% paraformaldehyde at 4°C for 24 hours, then shifted to 20% sucrose in 0.1 M PBS at 4°C for 48 hours; finally the brains were moved to a 30% sucrose solution kept at 4°C until sinking.
Coronal sections (10 μm) through the dorsal hippocampus were prepared using a freezing microtome (CM1950, Leica, Heidelberg, Germany). Every 12th section through the hippocampus was selected from each rat (Bregma −4.68 to −4.20 mm). The rabbit monoclonal anti-glial fibrillary acidic protein (GFAP) primary antibody (52kDa1:1000, Millipore) and rabbit anti-ionized calcium binding adapter molecule 1 specific protein (Iba-1, 1:200, Abcam) were used to measure astrocytic and microglial activation. The mouse anti-neuronal specific nuclear protein (NeuN, 1:600, Millipore) and the rabbit monoclonal p-CREB primary antibody (52kDa, 1:1000, CST) were used to detect neuronal damage. Sections were probed with the primary antibody at 4°C for 24 hours. After washing for three times, the sections were incubated with the secondary antibodies (anti-rabbit, Alexa 546; anti-mouse, Alexa 488, Molecular Probes, Cambridge, England) for 1 to 2 hours at room temperature. The sections were then observed under a fluorescent microscope (Olympus/BX51). Photomicrographs of CA1, CA3, and dentate gyrus (DG) subfields of the hippocampus (Hip) and prefrontal cortex (PFC) were taken using the 20× magnification of the fluorescent microscope. Two slices from the entire section of every rat brain were used for cell counting.
6. Tissue preparation and protein extraction
The rats were deeply anesthetized via 4% chloral hydrate, and then euthanized by cervical dislocation. The rats’ brains were quickly removed from the skull and placed into ice-cold PBS, and then the Hip was carefully dissected out for protein extraction. Total protein was extracted using tissue protein extraction reagent (Beyotime Institute of Biotechnology, China) containing EDTA-free complete protease inhibitors (Beyotime, China). The total protein concentration was determined using the Bio-Rad protein assay kit (Beyotime, China).
7. Enzyme-linked immunosorbent assay (ELISA)
Cytokines (TNF-α, IL-1β, and IL-6) in the rats’ Hip and PFT were measured using a Luminex kit (Youningwei, China). The procedures were as follows: all reagents were prepared, adding 50 μL of the standard or samples to each well, adding 50 μL of diluted microparticle cocktail to each well, and incubating for 2 hours at room temperature (RT) on a shaker at 800 rpm. Next, the liquid was removed from each well, wells were filled with 100 μL wash buffer, and the liquid again was removed. After performing the wash three times and adding 50 μL of diluted biotin-antibody cocktail to each well, covering, and incubating for 1 hour at RT on the shaker at 800 rpm, washing was repeated as before, adding 50 μL of diluted streptavidin-PE to each well, incubating for 30 minutes at RT on the shaker at 800 rpm, and repeating the wash again. Finally, 100 μL of wash buffer was added to each well, covered, and incubated for 2 minutes at RT on the shaker at 800 rpm. The results were read within 90 minutes using a Luminex analyser.
8. Western blot analysis
Western blot analysis was performed to detect the level of sEH, CD11b, CREB, and p-CREB in the PFC and Hip. Protein extracts were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to cellulose acetate membranes. After that, the membranes were blocked and incubated with primary antibodies including rabbit anti-sEH (63kDa, 1:500, ABclonal), rabbit anti-CREB (43kDa, 1:1000, CST), rabbit anti-CREB-phospho Ser133 (43kDa, 1:1000, CST), and rabbit anti-CD11b (127kDa, 1:500, NOVUS) at 4°C for 24 hours. The rabbit anti-β-actin primary antibody (40kDa, 1:1000, Beyotime) was used as an internal reference. Twenty-four hours later, the membrane was incubated with the goat anti-rabbit IgG secondary antibody (1:1000, Beyotime) for 2 hours at RT. Tanon Image software (version 4100, Shanghai, China) was used to analyse the bands of target proteins. The optical density (OD) value of each sample was normalised by the corresponding amount of β-actin.
9. Analyses of EETs in the rat brain tissue
The homogenate of rat hippocampus was centrifuged, and the supernatants were transferred to polypropylene tubes and stored at −20°C until analysis. The EETs levels were analysed by established liquid chromatography electrospray ionization tandem mass spectrometry method reported by Luo et al 30, 31.
10. Statistical analysis
Comparisons between groups were performed using the Student t test, one-way analysis of variance (ANOVA) test, or two-way ANOVA test depending on how many groups were included in the analysis. When using the one-way ANOVA test, a post-hoc Tukey test was adopted for comparisons between two groups. A P value of less than 0.05 was considered to be statistically significant. The data were expressed as mean ± standard error of the mean (SEM). The Graphpad Prism 7 software was used to conduct the statistical analysis in this study.
Results
1. TPPU shows anti-convulsant effects in the LiCl-pilocarpine-induced post-SE rat model
A total of 65 rats were used in this study, of which 12 rats were put in the control group, and 53 rats were given LiCl-pilocarpine to induce SE. Twelve of 53 rats died after SE; 11 of 53 rats were excluded because of no SRS was observed in the 2-week video monitoring after SE. Thus, 30 rats were included in the post-SE model, with 15 rats each in the SRS + PEG 400 and SRS + 0.1 TPPU groups according to randomised numbers.
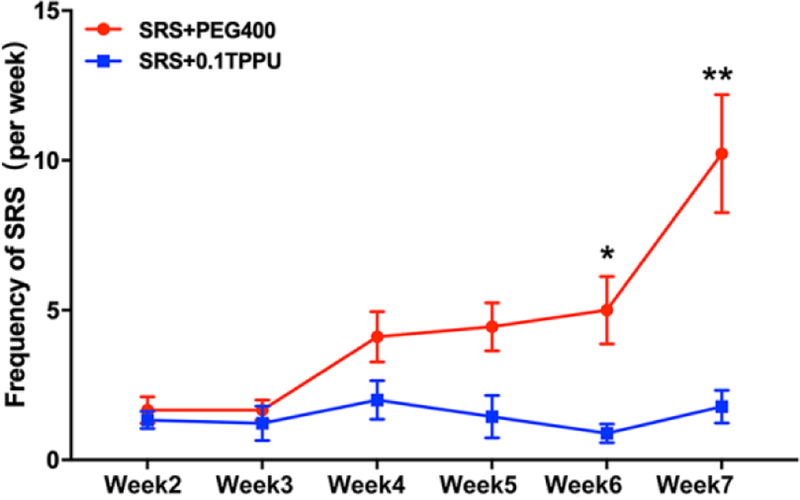
As spontaneous seizures in pilocarpine induced post-SE rats usually occurs in clusters with cyclicity of peaking every 5 to 8 days26, the number of seizures greater than or equal to Racine 3 degree was counted every week in this study. In the 2-week video monitoring after SE and before TPPU administration, no difference in the number of seizures was found between the SRS + PEG 400 and SRS + 0.1 TPPU groups. After TPPU administration, the seizure frequency of SRS every week was in a downward trend in the 0.1 TPPU group, which was significantly decreased at 21 days and 28 days after TPPU administration (equal to 6 w and 7 w after SE induction) compared with the SRS + PEG 400 group (at 21d, 0.89 ± 0.20 in TPPU group vs. 5.00 ± 1.13 in PEG400 group, P=0.0025; at 28d, 1.78± 0.55 in TPPU group vs. 10.22 ± 1.97 in PEG400 group, P=0.0008; n=9 in each group; see Fig.2, *P<0.05, **P<0.001).
Fig. 2.
The frequency of SRS shows a reduction after TPPU administration, with a significant decrease at 21d and 28d after TPPU administration (equal to 6 w and 7 w after SE induction) in the SRS + 0.1 TPPU group compared with the SRS + PEG 400 group, n = 9 in every group, *P<0.05, **P<0.001.
2. The anti-depressant effects of TPPU on the LiCl-pilocarpine-induced post-SE rat model
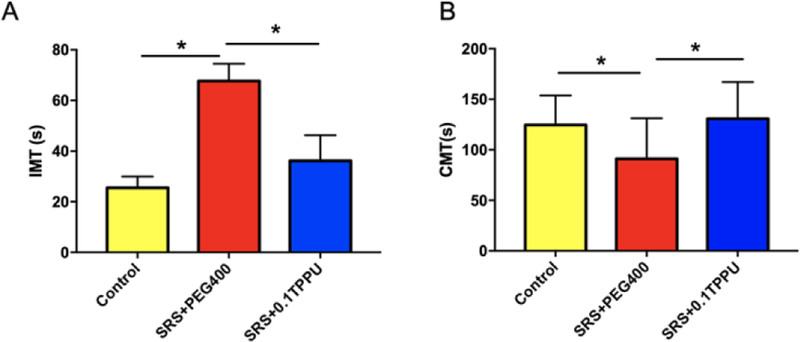
In the FST, the IMT, an indicator for despair, was significantly increased in the SRS + PEG 400 group compared with the control group (67.69 ± 6.79s vs. 25.50 ± 4.44s, P<0.001, n=13 in each group), which was significantly decreased after TPPU treatment (38.18 ± 11.95s in TPPU group, n=13, P=0.036, see Fig. 3A, *P<0.05, **P<0.01). Meanwhile, the CMT, an indicator for active behaviour, was significantly decreased in the SRS + PEG 400 group compared with the control group (91.15 ± 11.14s vs. 124.50 ± 9.26s, n=13 in each group, P=0.039), which was significantly increased after TPPU treatment (130.70 ± 10.11s, n=13, *p=0.015 see Fig. 3B, *P<0.05).
Fig. 3.
A) The IMT was significantly increased in the SRS + PEG 400 group compared with the Control group, and it was significantly decreased after TPPU treatment (*P<0.05, **P<0.01); B) The CMT was significantly decreased in the SRS + PEG 400 group compared with the Control group, and it was significantly increased after TPPU treatment (*P<0.05); n=13 in every group.
In the SPT, the SPR, an indicator for anhedonia, was in a declining trend after SE, and it had an upward trend after TPPU treatment, but without statistical differences between the SRS+PEG400 and SRS+0.1TPPU groups (data supplied in the supplementary materials).
3. The anti-inflammatory activity of TPPU in the PFC and Hip of the LiCl-pilocarpine-induced post-SE rat model
3.1. Astrocytic and microglial activation was attenuated by TPPU treatment
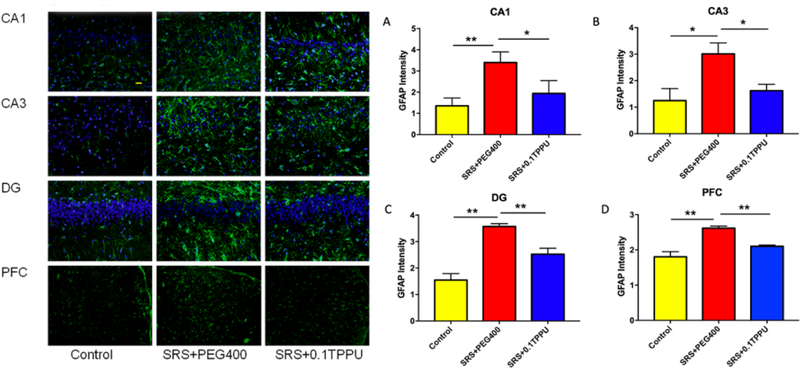
GFAP and Iba-1 are the markers for astrocytes and microglia in the brain, respectively. There was a significant astrocytic activation in the Hip and PFC of the SRS + PEG 400 group compared with the control group. Astrocytic gliosis in the CA1, CA3, and DG areas of the Hip and the PFC was significantly attenuated in the 0.1 TPPU group compared with the SRS + PEG 400 group (see Fig. 4A–D, n = 4 in every group, *P<0.05, **P<0.01).
Fig. 4.
The fluorescent intensity of GFAP-positive cells was significantly increased in the CA1 (A), CA3 (B), and DG (C) areas of the Hip and the PFC (D) in the SRS + PEG 400 group compared with the Control group. The astrocytic gliosis in the CA1, CA3, and DG areas of Hip and the PFC was all significantly attenuated after TPPU treatment (n = 4 in every group, *P<0.05, **P<0.01).
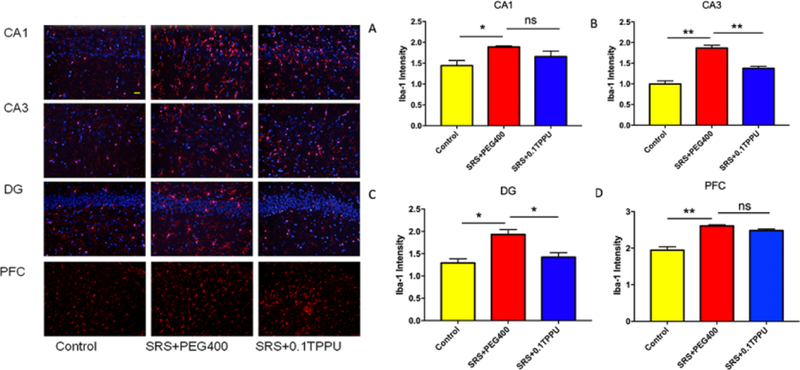
The fluorescent intensity of Iba-1 labelled microglia was significantly greater in the Hip and PFC of the SRS + PEG 400 group than the Control group (see Fig. 5A–D, n = 4 in every group, *P<0.05, **P<0.01); TPPU treatment significantly attenuated microglial activation in the CA3 and DG areas of the Hip compared with the SRS + PEG 400 group (Fig. 5B and 5C, *P<0.05, **P<0.01).
Fig. 5.
The fluorescent intensity of Iba-1-positive cells was significantly increased in the CA1 (A), CA3 (B), and DG (C) areas of the Hip and the PFC (D) of the SRS + PEG 400 group compared with the Control group. TPPU treatment significantly attenuated microglial gliosis in the CA3 (B) and DG (C) areas of the Hip (n = 4 in every group, *P<0.05, **P<0.01).
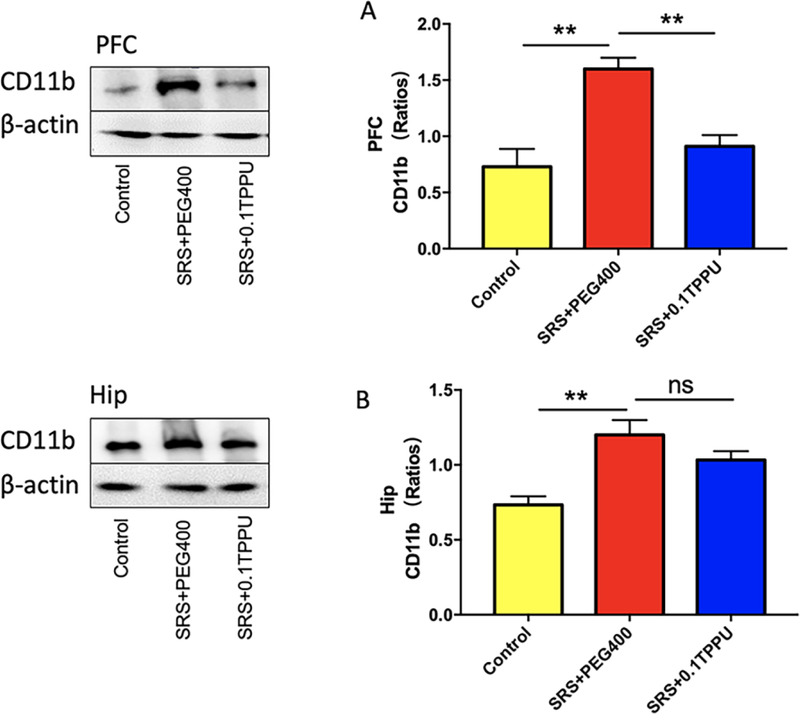
CD11b is another marker for microglia. The level of CD11b was significantly increased in the PFC and Hip of the SRS + PEG 400 group compared with the control group, and TPPU significantly attenuated the level of CD11b in the PFC compared with the SRS+PEG400 group (see Fig. 6A and 6B, n = 4 in every group, **P<0.01).
Fig. 6.
The expression of CD11b in the PFC (A) and Hip (B) was significantly increased in the SRS + PEG 400 group compared with the Control group, and it was significantly decreased in the PFC (A) after TPPU treatment, n = 4 in every group, *P<0.05, **P<0.01.
3.2. The levels of pro-inflammatory cytokines were decreased after TPPU treatment
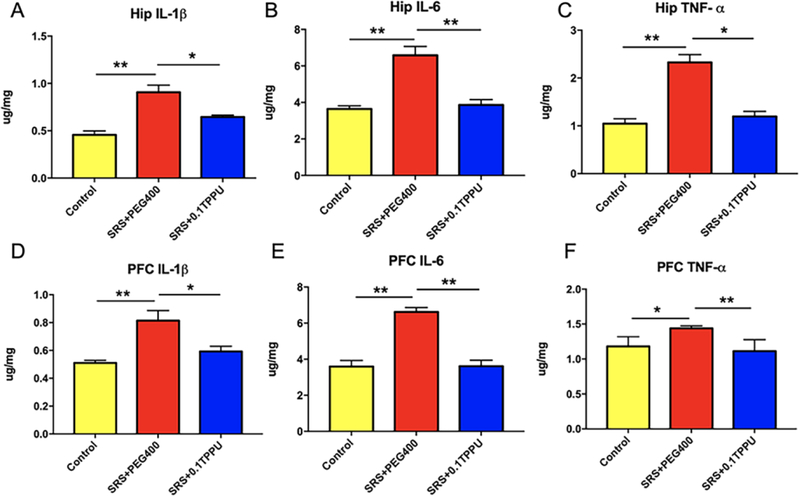
The ELISA method was used to determine the levels of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α in the PFC and Hip, which were significantly increased in the SRS + PEG 400 group compared with the control group (*P<0.05, **P<0.01). Treatment with TPPU significantly attenuated the high levels of IL-1β, IL-6, and TNF-α in the SRS + 0.1 TPPU group compared with the SRS + PEG 400 group (Fig. 7A–C for the Hip, Fig. 7D–F for PFC, n = 4 in every group, *P<0.05, **P<0.01).
Fig. 7.
The levels of the cytokines IL-1β, IL-6, and TNF-α in the Hip (A-C) and PFC (D-F) were significantly increased in the SRS + PEG 400 group compared with the Control group, and they were significantly decreased after TPPU treatment, n = 4 in every group, *P<0.05, **P<0.01.
3.3. The level of sEH in the PFC was decreased and the level of EETs was increased after TPPU treatment
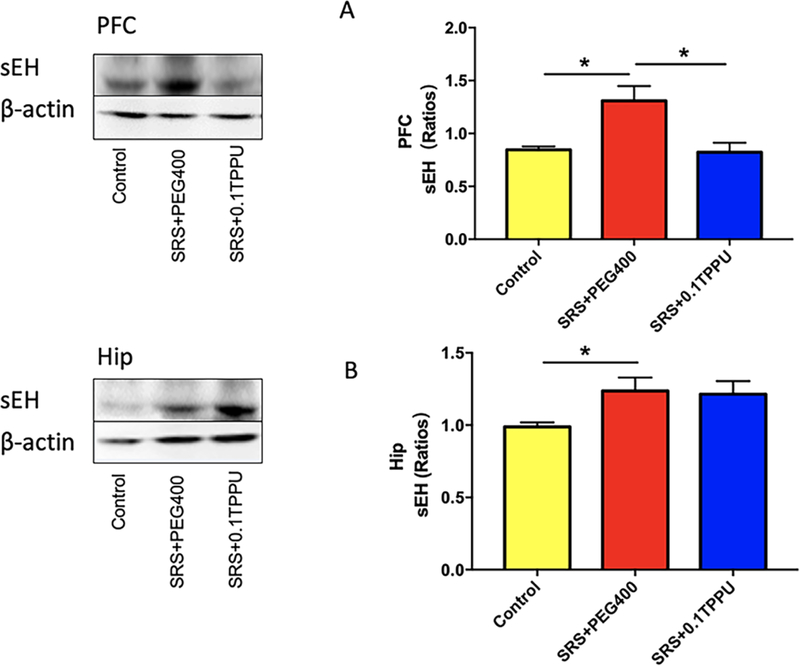
The level of sEH in the Hip and PFC was measured by western blot analysis. The result showed that the level of sEH was significantly greater in the Hip and PFC of the SRS + 0.1 TPPU group compared with the Control group (Fig. 8A and 8B, n = 4 in every group, *P<0.05). After TPPU treatment, the level of sEH in the PFC was significantly decreased in the SRS + 0.1 TPPU group compared with the SRS + PEG 400 group (Fig. 8A, *P<0.05).
Fig. 8.
The level of sEH in the PFC (A) and Hip (B) was significantly greater in the SRS + PEG 400 group than in the Control group, and it was significantly decreased in the PFC after TPPU treatment, n = 4 in every group, *P<0.05.
The level of EETs, the substrates of sEH, was also compared between the Control, SRS+PEG400, and SRS+0.1TPPU groups, which was significantly increased after TPPU treatment (127.24±11.29nmol/kg in Control vs. 108.32±12.64nmol/kg in PEG400 group vs. 190.85±27.83nmol/kg in 0.1TPPU group, n=7 in every group, p=0.022).
4. Both neuronal damage and the decreased expression of CREB in the Hip and PFC were attenuated after TPPU treatment
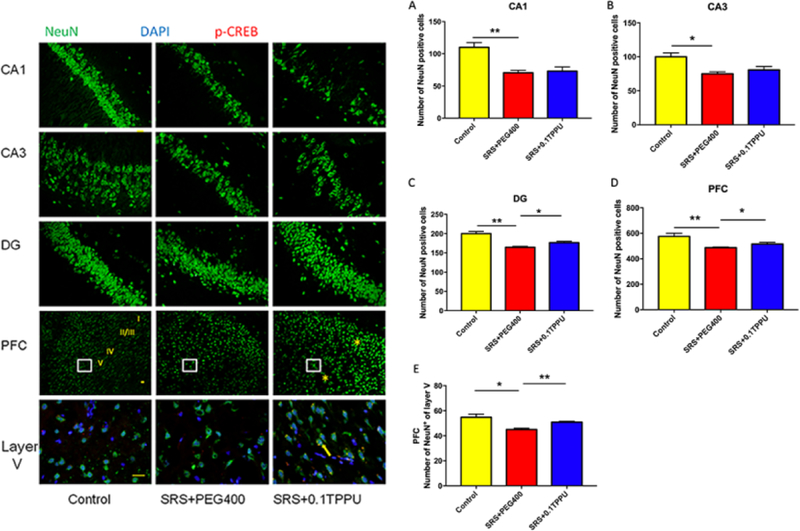
NeuN is a marker for healthy neurons. The number of NeuN-positive cells was compared between groups. A significant reduction of NeuN-positive cells in the CA1, CA3, and DG areas of the Hip and the PFC was observed in the SRS + PEG 400 group compared with the Control group (Fig. 9A–E, n = 4 in every group, *P<0.05, **P<0.01). After TPPU treatment, the number of NeuN-positive cells in the DG area (granular layer and hilus) of the Hip and the PFC including the layer V was significantly increased in the SRS + 0.1 TPPU group compared with the SRS + PEG 400 group (Fig. 9C–E, *P<0.05, **P<0.01). When triple-labelled NeuN with p-CREB and DAPI by the immunofluorescent method, it showed that the co-expression of the p-CREB and NeuN was increased in the layer V of PFC after TPPU treatment (Fig.9 Layer V of PFC).
Fig. 9.
The number of NeuN-positive cells in the CA1 (A), CA3 (B), and DG (C) areas of the Hip, the PFC (D), and the layer V of PFC (E) was all significantly decreased in the SRS + PEG 400 group compared with the Control group, *P<0.05, **P<0.01. TPPU treatment significantly attenuated the damage of NeuN-positive cells in the DG (C) area of the Hip and the PFC (D and E), n = 4 in every group, *P<0.05, **P<0.01. The co-expression of NeuN and p-CREB was increased after TPPU treatment as shown in the layer V of PFC (the yellow arrow shows that p-CREB is triple-labelled with NeuN and DAPI). 20× magnification micrographs are shown in the figures of CA1, CA3, DG, and PFC; 40× magnification micrographs are shown in the figure of layer V.
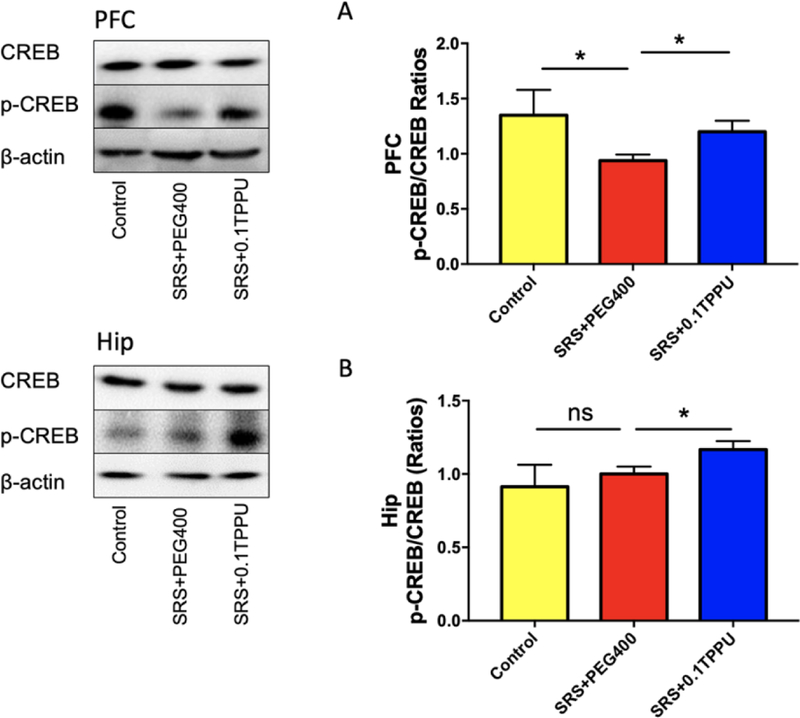
The ratio of p-CREB/CREB determined by western blot analysis was significantly decreased in the PFC of the SRS + PEG 400 group compared with the Control group (Fig.10A, n = 4 in every group, *P<0.05). After treatment with TPPU, the ratio of p-CREB/CREB in the PFC and Hip was significantly increased in the SRS + 0.1 TPPU group compared with the SRS + PEG 400 group (Fig. 10A and 10B, n = 4 in every group, *P<0.05).
Fig. 10.
The ratio of p-CREB/CREB in the PFC (A) was significantly decreased in the SRS + PEG 400 group compared with the Control group, and it was significantly increased in both of the PFC (A) and Hip (B) after TPPU treatment, n = 4 in every group, *P<0.05.
Discussion:
In this study, we investigated the anti-seizure and antidepressant effects of TPPU in the LiCl-pilocarpine-induced post-SE rat model. The LiCl-pilocarpine-induced post-SE model has been evaluated and verified to have depression-like behaviours, and was recommended as a model for the comorbidity of epilepsy and depression by Mazarati et al.32. The dose of TPPU was chosen based on previous experiments22 in our study. We found that using TPPU 0.1mg/kg/d for 4 weeks after SE not only attenuated the frequency of SRS but also alleviated the depression-like behaviours of the LiCl-pilocarpine-induced post-SE rat model.
The protective effects of TPPU on seizures have been verified in the picrotoxin, pentylenetetrazol, 6-Hz, maximal electroshock, and pilocarpine-induced seizure tests33. In addition, TPPU displayed rapid antidepressant effects on the inflammation and social defeat stress models of depression22. In our study, we found TPPU attenuated both spontaneous seizures and depression-like behaviours in the LiCl-pilocarpine-induced rat epilepsy model, with a major change of despair and a minor change of anhedonia, indicating a beneficial effect of TPPU on the epilepsy and its comorbidities, especially depression.
The sEH is the only intracellular oxidative enzyme responsible for hydrolysing EETs. It has a relative molecular weight of approximately 60 kDa and functions as a homodimer19. In the central nervous system, studies showed that sEH was extensively expressed in the cortical and hippocampal astrocytes and a few specific types of neurons in the cortex, cerebellum, and medulla of the mouse brain18. An in vivo study demonstrated that sEH was mainly localised in the cytoplasm, especially in and around the nucleus of the GFAP-positive astrocytes34. The EETs reduce neuronal apoptosis and promote nerve function recovery under pathological conditions17, whereas the increase of sEH has been found to be involved in some neurological diseases35. A study showed that sEH was increased in both lateral and medial temporal tissues of patients who underwent anterior temporal lobe resection due to TLE20, and was also found in the Hip of a pilocarpine-induced post-SE mouse model, co-expressed with GFAP-positive astrocytes, NeuN-positive neurons, and Iba-1-positive microglia36. In our study, we found that the expression of sEH was increased in both the Hip and PFC of the LiCl-pilocarpine-induced rat epilepsy model compared with normal control rats, which is consistent with these previous studies. TPPU treatment alleviated the high sEH level in the PFC but not in the Hip, which might be attributed by the mismatch of the protein level and enzyme activity22. The level of EETs, the substrates of sEH, in the Hip was significantly increased after TPPU treatment that also supported this point.
The mechanisms for the protective effects of sEHI on seizures and depression are not very clear. EETs are epoxide metabolites of cytochrome P450 (CYP) epoxygenases. In the brain, the endogenous EETs have important roles in cellular actions, regulation of cerebral blood flow, neurohormone release, and synaptic transmission16. As sEH is a key enzyme to hydrolyse bioactive EETs into DHET products that have reduced biological activity, inhibition of the activity of sEH may increase the effects of EETs. There was supporting evidence that injection of sEHI with EETs but not with epoxy-DHA or epoxy-EPA into the brains of mice delayed the onset of pentylenetetrazol-induced seizures33. In our study, we found that TPPU treatment attenuated astrocytic and microglial activation and the pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α in the Hip and PFC of the LiCl-pilocarpine rat epilepsy model, indicating the anti-inflammatory effects of sEHI. An increasing amount of evidences suggest that inflammatory processes are involved in epileptogenesis6. Hung et al. found that there were increased levels of the pro-inflammatory cytokines, IL-1β and IL-6, in the Hip of pilocarpine-induced SE mice, which persisted for at least 28 days after SE36. Our results supported this point. Simultaneously, we found not only in the Hip but also in the PFC that the cytokines including IL-1β, IL-6, and TNF-α were elevated in the LiCl-pilocarpine-induced post-SE rat model, indicating inflammatory mechanisms took a key role in the formation of epileptogenetic network of TLE, because the involved epilepsy network in TLE may contain mesial temporal areas and the PFC37. In addition, the astrocytic and microglial activation observed in our study supported that glial-mediated inflammation promoted neuronal hyperexcitability and epileptogenesis12. The anti-inflammatory effects of TPPU may have benefits against epileptogenesis. Our study indicated that treatment with TPPU after SE to suppress inflammation might contribute to ameliorate subsequent spontaneous recurrent epilepsy and its comorbidities.
The LiCl-pilocarpine-induced post-SE rat model has significant neuronal loss in the Hip and PFC in our study, which is in accordance with the previous studies38, 39. TPPU treatment alleviated the neuronal damage in both the Hip and PFC. The phosphorylation of CREB controls the induction and regulation of immediate-early genes that, in turn, induce the transcription of late downstream genes, and then activate effector proteins that are essential for neuronal survival, learning, and memory40. In our study, the ratio of p-CREB/CREB in the PFC significantly decreased in the epilepsy group compared with the control group, whereas it had no difference in the Hip, which might be attributed by the relatively less neuronal loss in the Hip in contrast with PFC demonstrated by the NeuN staining. TPPU treatment increased the expression of p-CREB/CREB ratio, further illustrating that the neuronal survival pathway was activated. Moreover, sEHI also has the ability to modulate mitochondrial dysfunction and endoplasmic reticulum stress41, which may also contribute to reducing neuronal damage.
In sum, we observed the effects of TPPU on the seizures and depressive behaviours in the LiCl-pilocarpine post-SE rat model. The limitation of this study is that we didn’t perform the intracranial electroencephalographic monitoring in the LiCl-pilocarpine rat epilepsy model that may lead to the non-convulsive seizures being missed. That will be the goal of our next experiment.
Conclusion
In this study, we demonstrated that treatment with TPPU after SE, a potent sEH inhibitor, attenuated subsequent SRS and epilepsy-associated depressive behaviours, and that TPPU took anti-inflammatory effects in the hippocampus and prefrontal cortex of the LiCl-pilocarpine-induced post-SE rat model. It indicates a new therapeutic method for epilepsy and its comorbidities, especially depression.
Highlights.
The inhibitor of soluble epoxide hydrolase TPPU attenuates spontaneous recurrent seizures and epilepsy-associated depression in the LiCl-pilocarpine-induced post-status epilepticus (SE) rat model
TPPU attenuates the inflammation and neuronal loss in the hippocampus and prefrontal cortex of the LiCl-pilocarpine-induced post-SE rat model
TPPU might be a new therapeutic approach for epilepsy and its comorbidities in the future, especially depression
Acknowledgments
Funding:
This work is supported by the project grant from the National Natural Science Foundation of China (81501114, 31771184), and it’s partially supported by NIEHS/Superfund Research Program P42 (ES004699) and NIH/U54 (NS079202).
Abbreviations
- AA
arachidonic acid
- CMT
climbing time
- COX
cyclooxygenase
- CREB
cAMP-response element binding protein
- CYP450
cytochrome P450 epoxygenases
- EETs
epoxyeicosatrienoid acids
- ELISA
Enzyme-linked immunosorbent assay
- ERK1/2
extracellular regulated protein kinase
- FST
Forced Swim Test
- GFAP
glial fibrillary acidic protein
- Hip
hippocampus
- Iba-1
ionized calcium binding adapter molecule 1 specific protein
- IL
interleukin
- IMT
immobility time
- i.p.
intraperitoneal
- LiCl
lithium chloride
- LOX
lipoxygenase
- NeuN
neuronal specific nuclear protein
- PFC
prefrontal cortex
- PEG
400polyethylene glycol 400
- PGE2
prostaglandin E2
- SE
status epilepticus
- sEH
soluble epoxide hydrolase
- sEHI
inhititor of soluble epoxide hydrolase
- SPR
Sucrose preference rate
- SPT
Sucrose preference test
- SRS
spontaneous recurrent seizures
- SSRIs
Selective serotonin reuptake inhibitors
- TLE
temporal lobe epilepsy
- TNF
tumor necrosis factor
- TPPU
1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests:
None of the authors has any conflict of interest related to this manuscript.
References
- 1.Josephson CB, Lowerison M, Vallerand I, et al. Association of Depression and Treated Depression With Epilepsy and Seizure Outcomes: A Multicohort Analysis. JAMA Neurol 2017;74:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 2016;15:106–115. [DOI] [PubMed] [Google Scholar]
- 3.Kanner AM. Can Neurochemical Changes of Mood Disorders Explain the Increase Risk of Epilepsy or its Worse Seizure Control? Neurochem Res 2017;42:2071–2076. [DOI] [PubMed] [Google Scholar]
- 4.Maguire MJ, Weston J, Singh J, Marson AG. Antidepressants for people with epilepsy and depression. Cochrane Database Syst Rev 2014;2014:CD010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mula M. Epilepsy and Psychiatric Comorbidities: Drug Selection. Curr Treat Options Neurol 2017;19:44. [DOI] [PubMed] [Google Scholar]
- 6.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013;69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler T, Li Y, Tsui W, et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia 2016;57:e191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 2008;22:797–803. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZH, Mong MC, Yang YC, Yin MC. Asiatic acid and maslinic acid attenuated kainic acid-induced seizure through decreasing hippocampal inflammatory and oxidative stress. Epilepsy Res 2018;139:28–34. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni SK, Dhir A. Cyclooxygenase in epilepsy: from perception to application. Drugs Today (Barc) 2009;45:135–154. [DOI] [PubMed] [Google Scholar]
- 11.Rojas A, Chen D, Ganesh T, Varvel NH, Dingledine R. The COX-2/prostanoid signaling cascades in seizure disorders. Expert Opin Ther Targets 2019;23:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devinsky O, Vezzani A, Najjar S, N.C. DL, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci 2013;36:174–184. [DOI] [PubMed] [Google Scholar]
- 13.Alyu F, Dikmen M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 2017;29:1–16. [DOI] [PubMed] [Google Scholar]
- 14.Mazarati AM, Pineda E, Shin D, Tio D, Taylor AN, Sankar R. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis 2010;37:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paudel YN, Shaikh MF, Shah S, Kumari Y, Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur J Pharmacol 2018;837:145–155. [DOI] [PubMed] [Google Scholar]
- 16.Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat 2010;91:68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Luo G, Zhang LF, Geng HX. Neuroprotective effects of epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat 2018;138:9–14. [DOI] [PubMed] [Google Scholar]
- 18.Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res 2009;1291:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris TR, Hammock BD. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene 2013;526:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmedov ML, Kemerdere R, Baran O, et al. Tissue Expressions of Soluble Human Epoxide Hydrolase-2 Enzyme in Patients with Temporal Lobe Epilepsy. World Neurosurg 2017;106:46–50. [DOI] [PubMed] [Google Scholar]
- 21.Vito ST, Austin AT, Banks CN, et al. Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute TETS intoxication. Toxicol Appl Pharmacol 2014;281:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Q, Ma M, Ishima T, et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci U S A 2016;113:E1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JY, Lin YP, Qiu H, et al. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci 2013;48:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng WF, Ding J, Li X, Fan F, Zhang QQ, Wang X. N-methyl-D-aspartate receptor NR2B subunit involved in depression-like behaviours in lithium chloride-pilocarpine chronic rat epilepsy model. Epilepsy Res 2016;119:77–85. [DOI] [PubMed] [Google Scholar]
- 25.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 26.Goffin K, Nissinen J, Van Laere K, Pitkanen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol 2007;205:501–505. [DOI] [PubMed] [Google Scholar]
- 27.Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem 2012;55:1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav 1993;54:1215–1220. [DOI] [PubMed] [Google Scholar]
- 29.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Wu MY, Deng BQ, et al. Inhibition of soluble epoxide hydrolase attenuates a high-fat diet-mediated renal injury by activating PAX2 and AMPK. Proc Natl Acad Sci U S A 2019;116:5154–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, Wang L, Peng A, Liu JY. Metabolic profiling of human plasma reveals the activation of 5-lipoxygenase in the acute attack of gouty arthritis. Rheumatology (Oxford) 2019;58:345–351. [DOI] [PubMed] [Google Scholar]
- 32.Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, Sankar R. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain 2008;131:2071–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inceoglu B, Zolkowska D, Yoo HJ, et al. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PloS one 2013;8:e80922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawal S, Morisseau C, Hammock BD, Shivachar AC. Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes. J Neurosci Res 2009;87:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther 2017;180:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hung YW, Hung SW, Wu YC, et al. Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun 2015;43:118–129. [DOI] [PubMed] [Google Scholar]
- 37.Spencer DD, Gerrard JL, Zaveri HP. The roles of surgery and technology in understanding focal epilepsy and its comorbidities. Lancet Neurol 2018;17:373–382. [DOI] [PubMed] [Google Scholar]
- 38.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 2008;172:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng WF, Fan F, Li X, Zhang QQ, Ding J, Wang X. Different behavioral and pathological changes between epilepsy-associated depression and primary depression models. Epilepsy Behav 2018;83:212–218. [DOI] [PubMed] [Google Scholar]
- 40.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 2009;89:121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediat 2017;133:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]