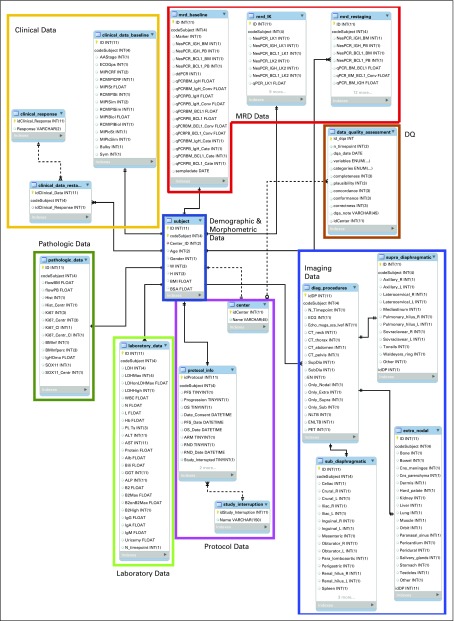
FIG 1.
Structure of the data warehouse (DW) for the collection of data recorded in the electronic case reporting forms (eCRFs) and in the data sources from laboratories during the clinical trial. The DW had a snowflake architecture. The subject table represented the center of the DW design and was directly connected to other categories: Protocol Data, Laboratory Data, Pathologic Data, Clinical Data, Imaging Data, and Minimal Residual Disease (MRD) Data tables. Three auxiliary tables were used in the Imaging Data category to encode the supra-diaphragmatic, sub-diaphragmatic, and extranodal involvements. The MRD Data category contained the information of both IgH and BCL1 biomarkers detected by both nested (qualitative) and real-time quantitative polymerase chain reaction in bone marrow (BM) and peripheral blood (PB) at baseline (mrd_baseline table); the MRD analysis was performed to monitor minimal residual disease on IgH and BCL1 markers from both BM and PB at each restaging (mrd_restaging table) and after the leukapheresis procedure (mrd_lk table). DQ, data quality.