Abstract
Purpose
The Controlling Nutritional Status (CONUT) score is a recently developed measure that is calculated using the serum albumin level, total cholesterol level, and lymphocyte counts. The aim of this study was to examine whether the CONUT score can predict post-operative outcomes in elderly patients undergoing curative gastrectomy.
Patients and methods
Pre-operative CONUT scores were evaluated from August 2014 to September 2016 in 357 gastric cancer patients who were scheduled to undergo curative gastrectomy. The patients were divided into three groups according to pre-operative CONUT scores: normal, light, moderate, and severe. We then calculated the association between the patient’s CONUT score and post-operative complications.
Results
CONUT scores were statistically associated with age (P = 0.015), body mass index (P < 0.001), pre-operative hemoglobin level (P < 0.001), tumor-node-metastasis stage (P < 0.001), surgical method (P = 0.036), and post-operative complications (P < 0.001). Multivariate analysis showed that age and the CONUT score were independent predictors of post-operative complications and 1-year survival.
Conclusion
CONUT scores can be used to predict post-operative complications and 1-year survival in elderly gastric cancer patients undergoing curative gastrectomy. They can also be used to classify the nutritional status of patients, which can be helpful for pre–and post-operative nutritional management.
Keywords: gastric cancer, nutrition, post-operative complications, CONUT score, elderly patients
Introduction
Gastric cancer is an aggressive neoplasm and is the third leading cause of cancer-related deaths worldwide.1 The treatment of gastric cancer continues to be a big challenge. Surgical resection is currently the main treatment modality in diagnosed patients.2 Gastrectomy is associated with several post-operative complications, such as infections, leakage, post-operative hemorrhage, delayed gastric emptying, and organ dysfunction. The presence of complications can lead to an increase in the length of post-operative recovery, with prolonged hospitalization and an increase in hospital costs.3
Malnutrition is a major concern for cancer patients, because it has a negative effect on malignancy progression, post-operative outcomes, response to anti-cancer treatment, hospitalization length, and cost.4 Controlling Nutritional Status (CONUT) score is a novel, simple evaluation measure that is calculated using serum albumin level, total cholesterol concentration, and total lymphocyte count measurement.5 Few studies have investigated the use of the CONUT score in cancer patients. To our knowledge, this is the first study investigating the role of the CONUT score in predicting post-operative outcomes in elderly gastric cancer patients undergoing curative gastrectomy.
Materials And Methods
Patients
In this prospective study, data of patients undergoing curative gastrectomy were collected between August 2014 and September 2016. The patients were treated following the Japanese guideline for treatment of gastric cancer. All patients had undergone standard D2 lymphadenectomy.6 The inclusion criteria were as follows: 1) proven gastric adenocarcinoma, 2) history of curative gastrectomy, 3) age ≥ 65 years, 4) no history of neoadjuvant treatment, and 5) no history of multiple organ resection. The study was approved by the ethics committee of The Second Affiliated Hospital of Wenzhou Medical University and complianced with the Declaration of Helsinki. Written Informed consent was obtained from all patients enrolled in this study.
Assessment Of CONUT Score
The pre-operative laboratory measurements included serum albumin level, total cholesterol concentration, and total peripheral lymphocyte count. The CONUT score was calculated as shown in Table 1, based on previous studies. The cut-off values were 35 g/L for serum albumin, 180 mg/dl for total cholesterol, and 1600/mm3 for total peripheral lymphocyte count.7,8 Patients with a score of ≥2 were considered to have malnutrition.5,9
Table 1.
Assessment Of Nutrition Status Based On CONUT Score
| Parameter | Degree Of Malnutrition | |||
|---|---|---|---|---|
| Normal | Light | Moderate | Severe | |
| Serum albumin (mg/dL) | > 35 | 30–34.9 | 25–29 | < 25 |
| Albumin score | 0 | 2 | 4 | 6 |
| Total Lymphocyte (/mL) | >1600 | 1200–1599 | 800–1199 | < 800 |
| Lymphocyte Score | 0 | 1 | 2 | 3 |
| Total Cholesterol (mg/dL) | > 180 | 140–180 | 100–139 | < 100 |
| Cholesterol Score | 0 | 1 | 2 | 3 |
| Total Score | 0-1 | 2–4 | 5–8 | 9–12 |
Data Collection
The data were collected from a prospectively maintained computer database. We retrieved data on the following demographic and clinicopathological features: age, sex, body mass index (BMI), hemoglobin concentration, diabetes, American Society of Anesthesiologists (ASA) grade, and tumor-node-metastasis (TNM) stage. We also retrieved the following surgical data: surgical method, surgery duration, type of gastrectomy (subtotal or total gastrectomy), type of anastomosis (Roux-En-Y, Billroth I, or Billroth II), and post-operative complications. The Clavien-Dindo classification method was used to classify post-operative complications and to avoid bias. Grade I complications were not analyzed in this study. No deaths were recorded in this patient group during the study period.
Statistical Analysis
SPSS Statistics software, version 22.0 (IBM Corporation, Armonk, NY, USA), was used for data analysis. Continuous variables following normal distribution were presented as mean and standard deviation (SD). Non-normally distributed variables were presented as median and interquartile range (IQR). Normally distributed and continuous variables were compared using the X2 test, while non-normally distributed variables were compared using the Mann–Whitney U-test. Univariate analysis was performed to find the potential risk factors, and multivariate analysis was then performed to identify independent predictors. A P-value < 0.05 was considered statistically significant.
Results
Patient Characteristics
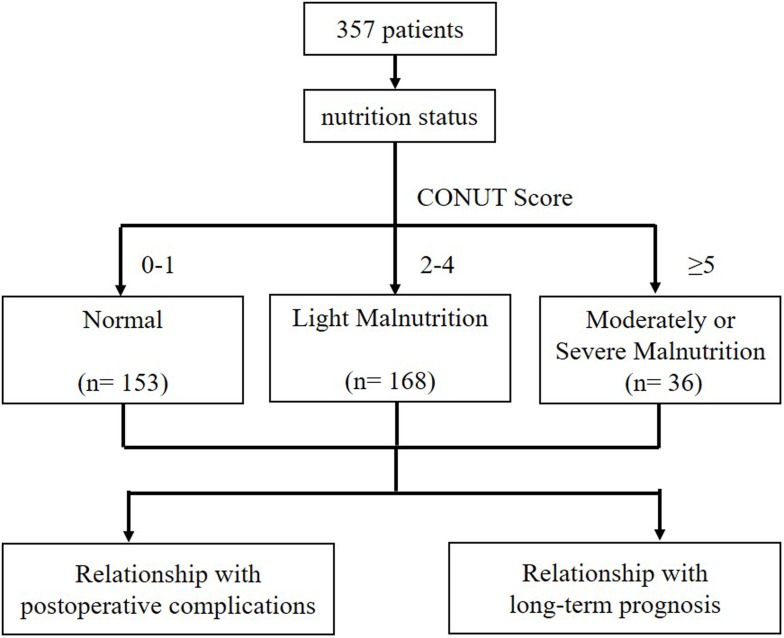
In the study, we enrolled 357 patients who met our inclusion criteria. According to the CONUT Score, we classified patients into three degrees: normal (0–1), light malnutrition (2–4), moderately or severe malnutrition (≥ 5). We analysed the correlations of nutrition status with Postoperative Complications and 1-year survival using logistic regression (Figure 1). Mean age of the patients was 73.29 ± 5.24 years. Most patients were male 275 (77%). Mean BMI of the patients was 21.61 ± 3.24, and 12.9% of patients had pre-operative diabetes. Mean pre-operative hemoglobin level was 107.2 ± 21.07. ASA grades of the included patients was as follows (in the descending order): II (245, 68.6%), III (86, 24.1%), I (24, 6.72%), and IV (2, 0.56%). TNM classification showed that most patients had stage III disease (151, 42.3%), followed by stage I (119, 33.3%) and stage II (87, 24.4%) disease. Regarding surgery, 79.3% of patients opted for open surgery, of which 56.9% underwent subtotal gastrectomy; the rest (43.1%) underwent total gastrectomy. In total, 47.1% of patients underwent Roux-En-Y anastomosis, 34.5% underwent Billroth I anastomosis, and the remaining 18.5% underwent Billroth II anastomosis. In most patients, the tumor location was the antrum (207, 58%), followed by the body (76, 21.3%), fundus (67, 18.8%), and pylorus (7, 1.9%). Mean surgery time was 202.6 ± 55.65 mins.
Figure 1.
Block flow chart of experimental grouping.
Association Of Clinicopathological Features With The CONUT Score
Statistical analysis of the association between the CONUT score and clinicopathological features showed that sex (P = 0.087), diabetes (P = 0.241), type of anastomosis (P = 0.063), type of gastrectomy (P = 0.393), tumor location (P = 0.086), and surgery time (P = 0.903) were not significantly associated with the CONUT score. However, we found that age (P = 0.015), BMI (P < 0.001), hemoglobin level (P < 0.001), TNM stage (P = 0.013), and surgical method (P = 0.036) were significantly associated with the CONUT score. We further analyzed the significant variables by performing a univariate analysis, to study their role as risk factors for post-operative outcomes (Table 2).
Table 2.
Clinicopathological Features Of Patients According To Nutritional Status
| Factors | Total | Normal (n= 153) | Light Malnutrition (n= 168) | Moderately Or Severe Malnutrition (n= 36) | P-Value |
|---|---|---|---|---|---|
| Age (years) | 73.29 (5.24) | 71.84 (4.77) | 72.20 (4.77) | 73.91 (5.79) | 0.015* |
| Gender | |||||
| Female | 82 | 41 | 33 | 8 | 0.087 |
| Male | 275 | 112 | 135 | 28 | |
| BMI | 21.61 (3.24) | 21.76 (3.42) | 22.16 (2.31) | 20.93 (2.94) | <0.001* |
| Diabetes | |||||
| No | 311 | 136 | 144 | 31 | 0.241 |
| Yes | 46 | 17 | 24 | 5 | |
| ASA grade | |||||
| I | 24 | 13 | 10 | 1 | 0.199 |
| II | 245 | 109 | 113 | 23 | |
| III | 86 | 31 | 43 | 12 | |
| IV | 2 | 0 | 2 | 0 | |
| Preoperation | 107.2 (21.07) | 127.4 (16.42) | 109.71 (19.9) | 95.47 (22.73) | <0.001* |
| Hemoglobin (IQR) | |||||
| TNM | |||||
| I | 119 | 64 | 49 | 6 | 0.013 |
| II | 87 | 33 | 44 | 10 | |
| III | 151 | 56 | 75 | 20 | |
| Surgical method | |||||
| Laparotomy | 283 | 114 | 137 | 32 | 0.036 |
| Laparoscopy | 74 | 39 | 31 | 4 | |
| Type of anastomosis | |||||
| Roux-en-Y | 168 | 69 | 83 | 16 | 0.063 |
| Billroth I | 123 | 62 | 50 | 11 | |
| Billroth II | 66 | 22 | 35 | 9 | |
| Type of gastrectomy | |||||
| Subtotal | 203 | 89 | 96 | 18 | 0.393 |
| Total | 154 | 64 | 72 | 18 | |
| Tumor location | |||||
| Fundus | 67 | 32 | 30 | 5 | 0.286 |
| Body | 76 | 30 | 40 | 6 | |
| Antrum | 207 | 90 | 92 | 25 | |
| Pylorus | 7 | 1 | 6 | 0 | |
| Surgery time (minutes) | 202.6 (55.65) | 203.2 (47.1) | 203.86 (57.70) | 196.81 (45.2) | 0.903 |
Notes: The values given are number of patients unless indicated otherwise. * Statistically significant (P< 0.05).
Abbreviations: BMI, body mass index; TNM, Tumor Node Metastasis; ASA, American Society of Anaesthesiologists; IQR, interquartile range.
Association Of Post-Operative Outcomes With The CONUT Score
Results of the statistical analysis for the association between the CONUT score and post-operative outcomes are shown in Table 3. The post-operative complications in our cohort were as follows: delayed gastric emptying (9 patients), ileus (12), pneumonia (21), anastomosis leakage (2), wound infection (4), anastomosis stenosis (2), ascites (7), deep venous thrombosis (3), pleural effusion (39), small bowel obstruction (7), lymph node leakage (2), pulmonary embolism (2), pleural effusion (39), intra-abdominal bleeding (5), intra-abdominal infection (19), septic shock (2), and multiple organ failure (19). Post-operative complications were significantly associated with the CONUT score (P < 0.001). Mean post-operative hospitalization length was 18.15 ± 10.12 days (P = 0.290); post-operative hospitalization length and lymph node metastasis (P = 0.132) were not significantly associated with the CONUT score. The CONUT score was significantly associated with 1-year survival.
Table 3.
The Relationship Between Postoperative Outcomes And Nutritional Status
| Factors | Total | Normal | Light Malnutrition | Moderate Or Severe malnutrition | P–value |
|---|---|---|---|---|---|
| Postoperative complications | |||||
| Clavien-Dindo Grade II | 96 | 41 | 44 | 11 | 0.535 |
| Delayed gastric emptying | 9 | 2 | 5 | 2 | |
| Ileus | 12 | 7 | 4 | 1 | |
| Pneumonia | 22 | 1 | 17 | 4 | |
| Anastomosis leakage | 2 | 1 | 1 | 0 | |
| Wound infection | 4 | 2 | 2 | 0 | |
| Anastomosis stenosis | 2 | 2 | 0 | 0 | |
| Ascites | 7 | 4 | 2 | 1 | |
| Deep venous thrombosis | 3 | 2 | 1 | 0 | |
| Small bowel obstruction | 7 | 5 | 0 | 2 | |
| Lymph node Leakage | 2 | 0 | 2 | 0 | |
| Pulmonary Embolism | 2 | 1 | 1 | 0 | |
| Pleural effusion | 39 | 5 | 30 | 4 | |
| Clavien-Dindo Grade III | 24 | 11 | 13 | 0 | 0.460 |
| Intra-abdominal bleeding | 5 | 2 | 3 | 0 | |
| Intra-abdominal infection | 19 | 9 | 10 | 0 | |
| Clavien-Dindo Grade IV | 2 | 1 | 1 | 0 | 0.674 |
| Septic shock | 2 | 1 | 0 | 1 | |
| Clavien-Dindo Grade V | 1 | 0 | 0 | 1 | 0.551 |
| Multiple Organ Failure | 1 | 0 | 0 | 1 | |
| Total complications | 113 | 29 | 68 | 16 | < 0.001 |
| Lymph Node Metastasis | 93 | 77 | 91 | 25 | *0.132 |
| Post-operative hospital stays (days) | 18.15 (10.12) | 15.69 (9.07) | 18.70 (10.78) | 17.92 (8.62) | 0.290 |
| 30–days readmission | 3 | 2 | 0 | 10 | 0.393 |
| One Year survival | |||||
| Alive | 331 | 149 | 152 | 30 | 0.002* |
| Dead | 26 | 4 | 16 | 6 | |
Notes: Data are expressed as number of patients, * Statistically significant (P< 0.05).
Univariate And Multivariate Analysis For Post-Operative Complications And 1-Year Survival
On univariate analysis, we found that age (P = 0.022) and the CONUT score (P < 0.001) were significant risk factors for post-operative complications. Subsequent multivariate analysis showed that age (P < 0.001) and the CONUT score (P < 0.001) were independent predictors of post-operative complications in our cohort (Table 4).
Table 4.
Univariate And Multivariate Analysis Of Factors Associated With Postoperative Complications
| Factors | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Complications | No Complications | OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Age | 74.45 (5.68) | 71.75 (4.81) | 1.105 | 1.057–1.155 | 0.022* | 1.094 | 1.045.-1.145 | < 0.001* |
| BMI | 21.95 (3.45) | 22.55 (3.13) | 0.944 | 0.880–1.012 | 0.347 | |||
| Hemoglobin | 113.60 (21.5) | 116.94 (21.88) | 0.993 | 0.983–1.003 | 0.561 | |||
| TNM | ||||||||
| I | 33 | 86 | ||||||
| II | 23 | 64 | 1.275 | 0.981–1.657 | 0.104 | |||
| III | 57 | 94 | ||||||
| Surgical Method | ||||||||
| Laparoscopy | 24 | 50 | 1.070 | 0.618–1.852 | 0.809 | |||
| Open | 89 | 194 | ||||||
| CONUT Score | ||||||||
| Normal | 29 | 124 | ||||||
| Light Malnutrition | 68 | 100 | 2.99 | 1.832–4.891 | < 0.001* | 2.695 | 1.631–4.451 | < 0.001* |
| Moderate/Severe Malnutrition | 16 | 20 | ||||||
Notes: *Statistically significant (P < 0.05), Data are expressed as number of patients.
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; BMI, Body Mass Index, CONUT Score, Controlling Nutritional Status.
Factors that could be associated with 1-year survival were analyzed by univariate and multivariate analysis. On univariate analysis, we found that age (P < 0.001), BMI (P = 0.044), TNM stage (P = 0.039), and the CONUT score (P = 0.030) were significant risk factors for 1-year survival. On multivariate analysis, we found that age (P < 0.001), TNM stage (P = 0.036), and the CONUT score (P = 0.021) were independent predictors of 1-year survival (Table 5).
Table 5.
Univariate And Multivariate Analysis Of Factors Associated With 1-Year Survival
| Factors | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Alive | Dead | OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| Age | 72.18 (4.96) | 78.00 (5.84) | 1.225 | 1.130–1.328 | < 0.001* | 1.214 | 1.116.-1.321 | < 0.001* |
| BMI | 22.45 (3.21) | 21.26 (3.52) | 0.900 | 0.802–1.010 | 0.044* | 0.967 | 0.845 −1.107 | 0.072 |
| Hemoglobin | 116.53 (21.76) | 107.65 (18.53) | 0.982 | 0.965–1.000 | 0.815 | |||
| TNM | ||||||||
| ≤ II | 196 | 10 | 2.232 | 1.023–5.274 | 0.039* | 2.398 | 0.982–5.853 | 0.036* |
| > II | 135 | 16 | ||||||
| Surgical Method | ||||||||
| Laparoscopy | 74 | 12 | 0.297 | 0.069–1.289 | 0.087 | |||
| Open | 257 | 24 | ||||||
| CONUT Score | ||||||||
| Normal | 149 | 4 | ||||||
| Light Malnutrition | 152 | 16 | 4.503 | 1.518–13.354 | 0.030* | 2.909 | 0.909–9.311 | 0.021* |
| Moderate/Severe Malnutrition | 30 | 6 | ||||||
Notes: *Statistically significant (P < 0.05), Data are expressed as number of patients.
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; BMI, Body Mass Index, CONUT Score, Controlling Nutritional Status.
Discussion
Patient’s nutrition, inflammation, and immune status can influence tumor progression.10,11 Surgical treatment is considered successful when there are no post-operative complications.12 Post-operative short-term outcomes and long-term survival in gastric cancer patients are of great concern for both surgeons and patients. It has been found that, compared with younger patients, elderly patients have later disease and poorer surgical tolerance, which are often associated with a worse long-term and short-term prognosis.13,14 Therefore, early identification of a population with poor post-operative prognosis could be important.
In the present study, we found that the CONUT score can be used as a predictor for post-operative complications and 1-year survival in elderly gastric cancer patients undergoing curative gastrectomy. The CONUT score is calculated from three parameters: serum albumin level, total cholesterol concentration, and peripheral lymphocyte count.15 Serum albumin is an indicator of protein reserves.16 Total peripheral lymphocyte count is an indicator of immunological status.17 Moreover, previous studies have found that T cells play a key role in the immune response against cancers.18 Menges et al19 found that lymphopenia is caused by a systemic inflammatory response resulting from a decrease in innate cellular immunity, which is indicated by a significant decrease in the number of T-4 helper lymphocytes and natural killer cells.19 A decrease in T cell count was shown to be correlated with poor prognosis because of inadequate host immunity against cancer.18 A low serum cholesterol level is associated with negative clinical outcomes in cancer patients.20,21 In cancerous tissues, there is an increased expression of the mRNA coding the low-density lipoprotein cholesterol receptor.22 This in turn increases the low-density lipoprotein cholesterol intake of the tumor tissue, causing a decrease in the serum cholesterol level.22 The cholesterol is used to accelerate tumor growth.23 This explains why cholesterol levels increase after surgery. A decrease in serum cholesterol level not only reflects a decrease in the caloric intake but also a decline in the cholesterol levels of the cell membrane, which is associated with a poor prognosis.24
Previous studies have shown that the CONUT score is associated with post-operative complications in colorectal cancer.25,26 Recently, Hirahara et al27 reported that the CONUT score is an independent predictor of survival in patients with esophageal cancer undergoing curative thoracoscopic esophagostomy. Furthermore, Tokunaga et al25 showed that the CONUT score predicts overall survival, relapse-free survival, and severe post-operative complications when patients are classified into three groups: normal, light, and moderate/severe CONUT score. To our knowledge, this is the first time that the CONUT score has been used to predict the long–and short-term prognosis in patients with gastric cancer. Except for TNM staging and tumor typing,28 the body’s nutritional state, inflammation, and the immune status are closely related to the disease’s prognosis.10,29 Peri-operative nutritional support in patients with malnutrition-based cancer can improve the nutritional status, enhance tolerance during treatment, and positively affect post-operative survival.30,31 Early identification and treatment of malnutrition by using the CONUT score in elderly patients undergoing curative gastrectomy may improve the surgical outcomes and reduce the post-operative complications.
This study has several limitations. First, a bias may exist, because the data were obtained from only a single institution. Second, although two researchers were responsible for data collection, artificial errors are unavoidable. Thus, a further validation, with larger, multi-center data sets, is needed to evaluate the role of the CONUT score in predicting the prognosis of gastric cancer patients.
Conclusion
The CONUT score is a simple, easy, and feasible score that reflects the nutritional and inflammatory status of a patient. Our study indicates that the CONUT score can help clinicians to predict post-operative complications and 1-year survival in elderly gastric cancer patients. Management of nutritional status may be crucial for survival in gastric cancer patients.
Acknowledgments
The authors thank all the participants in this study and the members of our research team.
Ethics Approval And Consent To Participate
All participants provided their written informed consent, and the protocol for this study was approved by the ethics committee of The Second Affiliated Hospital of Wenzhou Medical University and conformed to the tenets of the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.v68.6 [DOI] [PubMed] [Google Scholar]
- 2.Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17(1):43–53. doi: 10.1007/s10120-013-0234-1 [DOI] [PubMed] [Google Scholar]
- 3.Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95(13):e3164. doi: 10.1097/MD.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N, Baba Y, Shigaki H, et al. Preoperative nutritional assessment by Controlling Nutritional Status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. 2016;40(8):1910–1917. doi: 10.1007/s00268-016-3549-3 [DOI] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutricion Hospitalaria. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 8.Fukushima K, Ueno Y, Kawagishi N, et al. The nutritional index ‘CONUT’ is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J Exp Med. 2011;224(3):215–219. doi: 10.1620/tjem.224.215 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida N, Harada K, Baba Y, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2017;402(2):333–341. doi: 10.1007/s00423-017-1553-1 [DOI] [PubMed] [Google Scholar]
- 10.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto Y, Baba Y, Sakamoto Y, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015;10(6):e0129742. doi: 10.1371/journal.pone.0129742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100(8):1236–1239. doi: 10.1038/sj.bjc.6604997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelen SD, Bosscha K, Lemmens V, et al. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg. 2018;105(9):1163–1170. doi: 10.1002/bjs.10836 [DOI] [PubMed] [Google Scholar]
- 14.Song S, Li C, Li S, Cong X, Xue Y. Clinicopathological features and prognoses in younger and older patients with gastric cancer. Onco Targets Ther. 2017;10:4795–4802. doi: 10.2147/OTT.S144801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Madrono A, Mancha A, Rodriguez FJ, Culebras J, de Ulibarri JI. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: comparison with two logistic regression models developed using SGA as the gold standard. Nutricion Hospitalaria. 2012;27(2):564–571. doi: 10.1590/S0212-16112012000200033 [DOI] [PubMed] [Google Scholar]
- 16.Robinson R. Low serum albumin and total lymphocyte count as predictors of 30 day hospital readmission in patients 65 years of age or older. Peer J. 2015;3:e1181. doi: 10.7717/peerj.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: A systematic review and meta-analysis. Int J Surg. 2018;55:73–80. doi: 10.1016/j.ijsu.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Mella M, Kauppila JH, Karihtala P, et al. Tumor infiltrating CD8(+) T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology. 2015;4(6):e1002726. doi: 10.1080/2162402X.2014.1002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026 [DOI] [PubMed] [Google Scholar]
- 20.Ko K, Park YH, Lee JW, Ku JH, Kwak C, Kim HH. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int. 2013;112(6):775–780. doi: 10.1111/bju.2013.112.issue-6 [DOI] [PubMed] [Google Scholar]
- 21.Wettstein MS, Saba K, Umbehr MH, et al. Prognostic role of preoperative serum lipid levels in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate. 2017;77(5):549–556. doi: 10.1002/pros.v77.5 [DOI] [PubMed] [Google Scholar]
- 22.Gabitova L, Gorin A, Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res. 2014;20(1):28–34. doi: 10.1158/1078-0432.CCR-13-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol. 2013;4:119. doi: 10.3389/fphar.2013.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Ulibarri Perez JI, Fernandez G, Rodriguez Salvanes F, Diaz Lopez AM. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutricion Hospitalaria. 2014;29(4):797–811. doi: 10.3305/nh.2014.29.4.7275 [DOI] [PubMed] [Google Scholar]
- 25.Tokunaga R, Sakamoto Y, Nakagawa S, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32(1):99–106. doi: 10.1007/s00384-016-2668-5 [DOI] [PubMed] [Google Scholar]
- 26.Iseki Y, Shibutani M, Maeda K, et al. Impact of the preoperative Controlling Nutritional Status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS ONE. 2015;10(7):e0132488. doi: 10.1371/journal.pone.0132488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther. 2018;25(5):e524–e532. doi: 10.1097/MJT.0000000000000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–150. doi: 10.1200/JCO.2015.61.6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali Abdelhamid Y, Chapman MJ, Deane AM. Peri-operative nutrition. Anaesthesia. 2016;71(Suppl 1):9–18. doi: 10.1111/anae.2016.71.issue-S1 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Xue X. Systematic review of peri-operative nutritional support for patients undergoing hepatobiliary surgery. Hepatobiliary Surg Nutr. 2015;4(5):304–312. doi: 10.3978/j.issn.2304-3881.2014.12.09 [DOI] [PMC free article] [PubMed] [Google Scholar]