Abstract
Microfluidics brings unique opportunities for engineering micro-/nanomaterials with well-controlled physicochemical properties. Herein, using a miniaturized multi-run spiral-shaped microreactor, we develop a flow synthesis strategy to continuously produce hollow spherical silica (HSS) with hierarchical sponge-like pore sizes ranging from several nanometers to over one hundred nanometers. The formation of HSS is realized by mixing two reactant flows, one containing cetyltrimethylammonium bromide (CTAB) and diluted ammonia and the other 1,3,5-trimethylbenzene (TMB) and diluted tetraethyl orthosilicate (TEOS), at a flow rate as high as 5 mL/min. The effect of the reactant concentration and the flow rate on the structural change of the resultant materials is examined. Functional small-sized nanoparticles (magnetic nanoparticle, quantum dot, and silver nanoparticle) can be separately assembled into HSS and high molecular weight protein (bovine serum albumin) can be successfully loaded into HSS and delivered into cancer cells afterward, making them promising in the fields of separation and purification, bioimaging, catalysis, and theranostics.
Keywords: microfluidic, shape, mesoporous silica, large pore, sponge-like
Graphical abstract
1. Introduction
The last two decades have witnessed unprecedented advances in the ability to synthesize new porous silica materials since their discovery in the early 1990s.[1] Given their unique physicochemical features, especially large surface area, high pore volume, robust mechanical and thermal stability, and good biocompatibility, porous silica materials provide great potentials across a wide variety of applications such as electronics, separation and purification, sensors, bioimaging, catalysis, and biomedicine. To date, porous silica materials with various particle sizes, shapes, and surface functionalities have been successfully developed for meeting the needs of these specific application fields. [2] However, it is noted that the pore sizes of mostly porous silica materials are usually very small (ca. 1-5 nm) resulting from the use of alkylammonium surfactants as templates, [3] which limits their applications for which significant mass transport of large-sized molecules/particles is essential. Therefore, silica supports with easily accessible large porous structure are greatly needed. In recent years, two main approaches have been developed to produce large porous silica materials. One is to adopt high molecular weight block polymer and the other is to introduce a pore-swelling agent such as alkanes and alkylbenzenes.[4] However, the former usually results in the porous silica products displaying irregular morphologies, while the latter is only applicable for slightly increasing the pore size through conventional batch reaction platforms. There is still a great challenge to produce well-defined porous silica materials with controlled pore size of tens of nanometers or even hundred nanometers.
Microfluidics as a cutting-edge technique for engineering micro-/nanomaterials permits well-controlled physicochemical properties. [5–12] Compared with conventional batch reactors, microfluidics-based microreactors provide many unique and appealing features. These include but are not limited to: 1) greatly reduced mixing time of chemical reactants down to the order of microseconds; 2) rapid reaction kinetics for fast screening of experimental parameters; 3) intensive and sufficient mixing inside the microchannels for high yields; 4) large surface-to-volume ratio of microchannels for enhanced heat and mass transfer; 5) confining active starting materials into a small space for offering great chances to create new materials; 6) automatic operations for minimizing local variations and easy scaling-out. Therefore, microfluidic reactors may shed new lights on the synthesis of large porous silica materials.
In this study, using a miniaturized spiral-shaped microfluidic reactor with one inlet flow containing cetyltrimethylammonium bromide (CTAB) and diluted ammonia and the other inlet flow containing 1,3,5-trimethylbenzene (TMB) and diluted tetraethyl orthosilicate (TEOS), we first developed a continuous and scalable flow synthesis strategy to fabricate hollow spherical silica (HSS) with hierarchical sponge-like large porous shell structure. The effect of the reactant concentration and the flow rate on the structural changes of the resultant materials was examined. We further investigated the possibilities of one-step synthesis of multifunctional HSS with specific magnetic, fluorescent, or catalytic properties and high molecular weight protein loading for intracellular delivery.
2. Experimental
Details related to the chemical reagents used are presented in the text of Supporting Information. All experiments were conducted at room temperature unless otherwise indicated. The 2-run microfluidic spiral channel with two inlets and one outlet was fabricated using polydimethylsiloxane (PDMS) through soft lithography. Microchannels were formed by bonding the PDMS replica to a standard glass slide after oxygen plasma treatment. The spiral-shaped microchannel is made of three arcs with the diameters of 7.69, 13.8, and 22.2 mm, and with the central angles of 180°, 180°, and 225°, respectively. The height and width of the microchannel are 50 and 500 μm, respectively. The synthesis of HSS was realized simply with one inlet containing CTAB (13.7 mM) in diluted ammonia (0.7 M) and the other containing TMB (0.6 M) in diluted TEOS (0.3 M). The two inlet flows were pumped (Pump 33 DDS, Harvard Apparatus) into the spiral microchannel at a same flow rate of 1 mL/min. The as-synthesized products were collected at the outlet. Details related to the simulation, additional characterization, and other multifunctional materials synthesis results are presented as Supporting Information.
3. Results and discussion
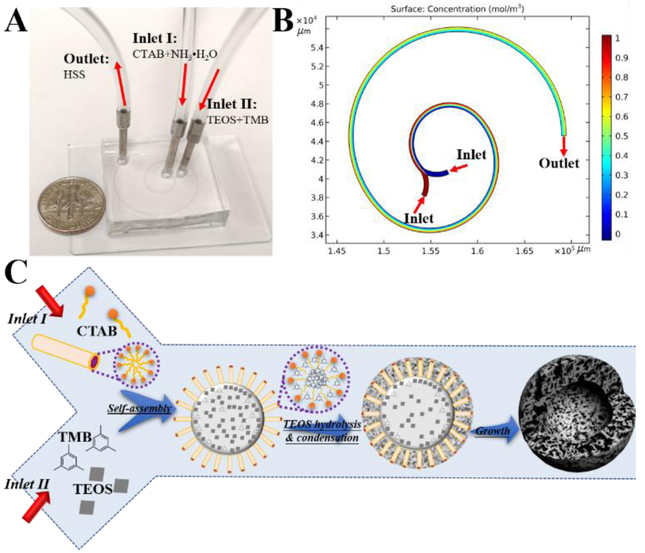
Microfluidic flow synthesis of HSS was carried out in the two-run spiral-shaped microreactor with two inlets and one outlet (Figure 1A). The microfluidic device was fabricated using soft lithography technique (Figure S1). In brief, polydimethylsiloxane (PDMS) precursor was poured over the master mold and peeled off to get the replica after solidification. The PDMS replica was subsequently treated by oxygen plasma and bonded to the glass slide to form the microchannels (See details in SI). The spiral-shaped microchannel is made of three arcs with the diameters of 7.69, 13.8, and 22.2 mm, and with the central angles of 180°, 180°, and 225°, respectively. The height and the width of the microchannel are 50 and 500 μm, respectively. The two inlet reactant flows, one containing CTAB (13.7 mM) in diluted ammonia (0.7 M) and the other containing TMB (0.6 M) in diluted TEOS (0.3 M), were pumped (Pump 33 DDS, Harvard Apparatus) into the microreactor both at the flow rate of 1 mL/min at room temperature. The porous silica product was collected from the outlet. Due to the presence of transverse Dean flow, spiral-shaped microreactors give rise to rapid and efficient mixing,[13–16] as further revealed by the COMSOL simulation analysis (Figure 1B). On the basis of these merits, the formation of large porous silica materials can be thus realized by the ammonia-catalyzed hydrolysis and condensation of TEOS using CTAB as the pore-forming agent and TMB as the pore-swelling agent in the microchannel, where the TEOS droplets were confined by TMB-embedded CTAB micelles (Figure 1C).
Figure 1.
Microfluidic flow synthesis of hollow spherical silica (HSS) with hierarchical Sponge-like Porous structure. (A) Experimental setup for the synthesis of HSS, with a U.S. one dime coin for scale. (B) Simulation result of mixing in spiral-shaped microchannel. (C) Schematic drawing for showing the formation of HSS (objects are not drawn to scale).
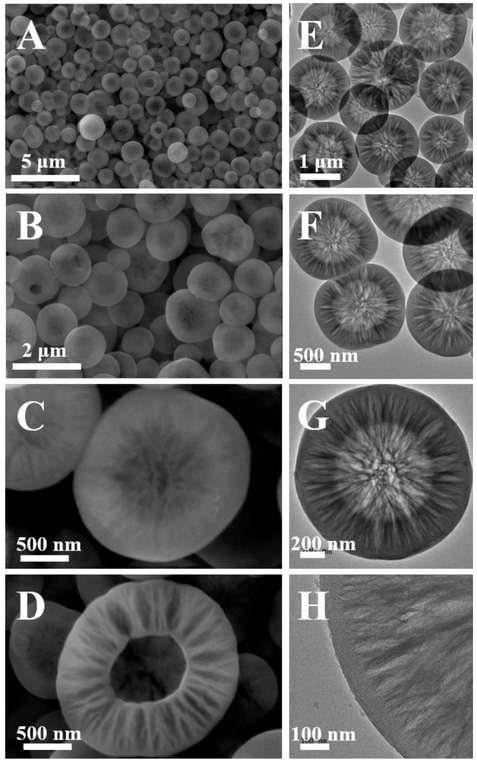
Given the superior merits of the microreactors, we expect that the desired large porous silica could be quickly and continuously produced over a short distance of microchannels (<8 cm in length). When the flow rate of the two inlet fluids was set as 1 mL/min, white colloidal suspension could be observed within 120 ms from the outlet (see details in SI). As shown in figure 2A, well-defined spherical silica particles having an average diameter of ca. 1.2 μm (1254 ± 237 nm, Figure S2) can be clearly identified under the SEM. From the high magnification SEM images (Figures 2B-D), the abundant porous channels can be obviously seen on the particle surface (Figures 2B-C), and a typical hollow core and sponge-like large porous shell structure was observed from an incomplete closure of the silica particles (Figure 2D). Observations from TEM images not only confirm the presence of hollow core-shell structure of the silica product from the microreactor, but also reveal richer porous texture of HSS with hierarchical pore size ranging from several nanometers to over one hundred nanometers (Figures 2E-H). Nitrogen sorption analysis was further performed to provide evidence of the porous properties of HSS (Figure S3). The results showed that HSS exhibits a typical type IV isotherm with H3-type hysteresis loop in the high relative pressure range of 0.90-0.95, indicating the presence of slit-shaped large pores (Figure S3A).[4,17,18] An evident hysteresis loop and capillary condensation step can be also observed at the relative pressure range of 0.2-0.8, suggesting the wide pore size distributions in HSS (Figure S3A).[17,19] The multiple pore size distributions ranging from several nanometers to over 100 nm calculated by density functional theory (DFT) method are well in agreement with the SEM and TEM measurements (Figure S3B). In addition, the specific surface area and the total pore volume of HSS are about 481.5 m2/g and 0.72 cm3/g, respectively.
Figure 2.
Scanning electron microscopy (SEM, A–D) and transmission electron microscopy (TEM, E–H) images of as-synthesized porous HSS at different magnifications.
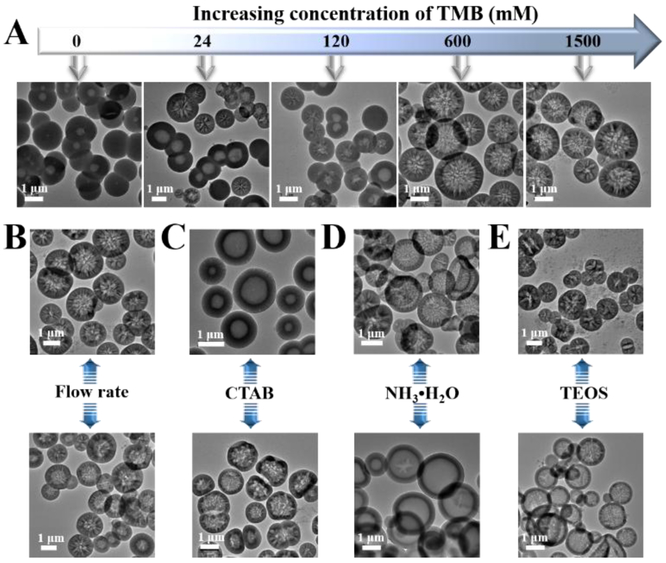
To explore the formation mechanism and the tunability of HSS from such two-run spiral-shaped microreactor, we further investigated the roles of reaction parameters, including TMB, flow rate, CTAB, ammonia, and TEOS, in the morphology change of the porous silica products. As shown in figure 3 A, the presence of TMB plays an important role in the formation of large porous particles. Without the addition of TMB, only common hollow spherical particles with mesoporous shell (pore size<5 nm) were formed, which is in good agreement with our recent findings of CTAB-aided one-step synthesis of hollow core-shell structures. [20,21] However, when relatively low amounts of TMB (24 or 120 mM)were added, hollow silica particles with a pore size ranging from several nanometers to over 100 nm can be observed, though there still remain some hollow silica particles with small-sized pores in the shell (pore size<5 nm). If continuously increasing the concentrations of TMB (600 mM or above), hollow spherical silica particles with hierarchical sponge-like large porous shell structure were obtained in a large scale. Comparatively, the flow rate of inlet fluids plays minor effects on the structural morphology, well-defined large porous HSS can be still obtained when decreasing the flow rate to 50 μL/min or increasing it to 5 mL/min (Figure 3B). In addition, hollow silica with large porous shell structure can be still maintained when decreasing the concentration of CTAB (5.5 mM) or increasing the concentration of ammonia (2.1 M), whereas, hollow silica with small-sized mesopores in the shell (pore size<15 nm) was obtained when increasing the concentration of CTAB (41.1 mM) or decreasing the concentration of ammonia (0.28 M, Figures 3C-D). The concentration of TEOS plays a main role in the regulation of the shell thickness. Increasing the concentration of TEOS (0.9 M) resulted in thicker shell porous silica (~500 nm shell thickness) and some nanosized silica (particle size<20 nm) byproducts, while decreasing the concentration of TEOS (0.1 M) could yield hollow porous silica with a thinner shell (~100 nm shell thickness, Figure 3E). These results not only confirmed the role of CTAB as pore-forming agent, but also revealed the important role of TMB as pore-swelling agent during the formation of HSS.
Figure 3.
Effect of reaction conditions, including the concentration of TMB (A), the flow rate of fluids (B), the concentration of CTAB (C), the concentration of ammonia (D), and the concentration of TEOS (E), on the morphology change of silica products.
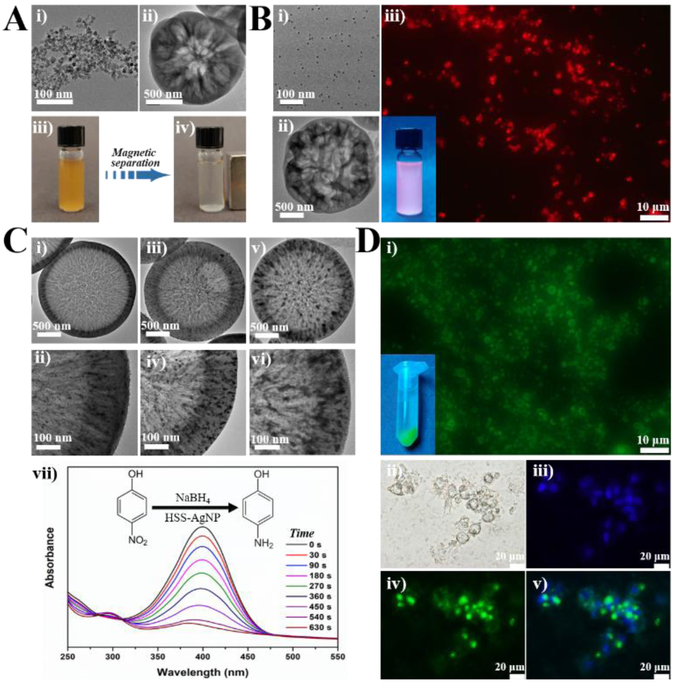
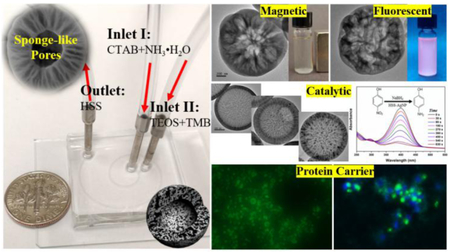
Considering the ease of operation, tunability, and scalability of microreactors, the feasibility of microfluidic one-step flow synthesis of multifunctional large porous silica was further examined. Different types of functional small-sized nanoparticles, including magnetic nanoparticle (MNP), quantum dot (QD), and silver nanoparticle (AgNP), were chosen to be simultaneously assembled into HSS, endowing it with specific magnetic, fluorescent, or catalytic properties. The capability of high molecular weight bovine albumin-fluorescein isothiocyanate conjugate (BSA-FITC) loading by HSS and its intracellular protein delivery performance was also investigated. For the synthesis of magnetic HSS, Fe3O4 nanoparticles having an average size of ca. 10 nm were firstly fabricated via coprecipitation method[22] (Figure 4Ai) and then added into the inlet flow of CTAB and ammonia (Inlet I). The resultant HSS-MNP still exhibit typical hollow structure with sponge-like large pores on the shell (Figures 4Aii & S4). When placing an external magnet, HSS-MNP particles can be separated rapidly from suspension (ca. 15 s, Figures 4Aiii-iv), which further confirms the successful and efficient one-step encapsulation of MNP into the porous silica matrix. Similarly, we added CdSe@ZnS QDs, having an average size of ca. 5 nm (Figure 4Bi),[23] to the Inlet I fluid and successfully obtained HSS-QD with large porous hollow core-shell structure in a one-step flow synthesis strategy (Figure 4Bii). The bright red color of HSS-QD over one month of shaking treatment in water at room temperature further demonstrated that QDs can be firmly assembled into the porous silica matrix (Figure 4Biii). To integrate AgNP into HSS, AgNO3 was added into the Inlet I fluid and the product collected from the outlet was then calcinated to obtain HSS-AgNP. To further test the tunability of this microreaction system, three different concentrations of AgNO3 (35, 70, and 140 mM) were used. Results showed that AgNP can be effectively and uniformly distributed across the shell of hollow porous silica (Figures 4Ci-vi). The loading amount of AgNP from HSS-AgNP was increased from 8.9% (Figures 4Ci-ii & S5) to 15.2% (Figures 4Ciii-iv & S6) to 27.1% (Figures 4Cv-vi & S7) with the increasing concentration of AgNO3. The catalytic performance of reduction of 4-nitrophenol (4-NP) into 4-aminophenol (4-AP) in the presence of NaBH4 using HSS-AgNP was then evaluated (Figure 4Cvii). When adding HSS-AgNP into the mixture of 4-NP and NaBH4, the adsorption peak of 4- NP at 400 nm decreased quickly and the solution color changed from yellow to colorless (Figure 4Cvii), indicating the effective reduction of 4-NP to 4-AP.[24–26] The kinetic reaction rate constants were calculated to be 9.8 × 10−3 s−1, 6.1 × 10−3 s−1, and 4.7 × 10−3 s−1 by the samples from figure 4Ci, figure 4Ciii, and figure 4Cv, respectively. In addition, the structures of HSS-AgNP can be still well maintained after eight cycles of catalysis reaction (Figure S8). To test the loading capability of high molecular weight protein by HSS, BSA-FITC having an estimated size dimension of 14 × 4 × 4 nm was chosen as a model protein. [27,28] As shown in figure 4Di, the bright green color from the solid particles reveals that BSA-FITC can be efficiently loaded into HSS, and the protein loading amount of HSS-BSA-FITC was calculated to be 387.5 mg per gram porous silica material. After treated with HSS-BSA-FITC for 2 h, MDA-MB-231 cells showed recognizable green color, indicating the successful cellular uptake and intracellular protein delivery via HSS as a carrier (Figures 4Dii-v). These results demonstrated that such two-run spiral-shaped microreactor not only allows one-step synthesis of functional large porous hollow silica with specific magnetic, fluorescent, or catalytic properties, but also makes the resultant HSS promising carriers for intracellular protein delivery.
Figure 4.
Microfluidic synthesis of HSS-based functional materials. (A) TEM image of Fe3O4 MNP (i) used for the one-step synthesis of HSS-MNP (ii); (iii) magnetic separation behavior of HSS-MNP under an external magnet. (B) TEM image of CdSe@ZnS QD (i) used for the one-step synthesis of HSS-QD (ii); (iii) fluorescent image of HSS-QD under fluorescent microscope and the inset is a photo of HSS-QD solution under irradiation of an UV lamp. (C) TEM images of HSS-AgNP with different loading amounts of Ag (i, iii, and v) and their corresponding high magnification images (ii, iv, and vi); (vii) representative time-dependent UV-vis spectral changes of 4-nitrophenol catalyzed by the HSS-AgNP from (i). (D) Fluorescent images of HSS loaded with BSA-FITC (i) and the inset is a photo of HSS-BSA-FITC solution under irradiation of an UV lamp; (ii-iv) bright field and fluorescent images of MDA-MB-231 cells treated with HSS-BSA-FITC for 2 h, nuclei were counterstained with Hoechst 33342; a merged image is shown in (v).
4. Conclusions
In summary, we first developed a continuous and scalable flow synthesis strategy to fabricate large porous hollow core-shell silica material using a two-run spiral-shaped microfluidic reactor with one inlet flow containing CTAB and diluted ammonia and the other inlet flow containing TMB and diluted TEOS. HSS exhibited typical hierarchical sponge-like porous structure with pore size ranging from several nanometers to over one hundred nanometers. The formation of HSS can be realized with a pump-driven flow rate of as high as 5 mL/min. The effect of the reactant concentration and the flow rate on the structural changes of the resultant materials was examined. Different types of functional small-sized nanoparticles, including MNP, QD, and AgNP, were simultaneously assembled into HSS and endow it with specific magnetic, fluorescent, or catalytic properties. High molecular weight BSA-FITC was loaded into HSS for achieving successful intracellular protein delivery. These results not only shed new lights on the rational design of smart micro-/nanostructures, but also provide a general platform for producing multifunctional materials in separation and purification, bioimaging, catalysis, and theranostics fields.
Supplementary Material
Highlights.
Spiral shaped laminar flow microreactor for large porous materials synthesis
Hollow core-shell silica with hierarchical large pore sizes was first reported
Particle can be formed with a flow rate of as high as 5 mL/min
Different type of nanoparticles can be simultaneously assembled into silica matrix
High molecular weight protein can be loaded for intracellular delivery
Acknowledgements
This work was sponsored by the NIH Director’s Transformative Research Award (R01HL137157), and NSF grants (ECCS 1128677, 1309686, 1509369). We gratefully acknowledge the support from the Electron Microscope Facility at Dartmouth College.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS, Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism, Nature. 359 (1992) 710–712. doi: 10.1038/359710a0. [DOI] [Google Scholar]
- [2].Hao N, Li LF, Tang FQ, Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems, Int. Mater. Rev. 62 (2017) 57–77. doi: 10.1080/09506608.2016.1190118. [DOI] [Google Scholar]
- [3].Polshettiwar V, Cha D, Zhang X, Basset JM, High-surface-area silica nanospheres (KCC-1) with a fibrous morphology, Angew. Chem. Int. Ed. 49 (2010) 9652–9656. doi: 10.1002/anie.201003451. [DOI] [PubMed] [Google Scholar]
- [4].Niu D, Liu Z, Li Y, Luo X, Zhang J, Gong J, Shi J, Monodispersed and ordered large-pore mesoporous silica nanospheres with tunable pore structure for magnetic functionalization and gene delivery, Adv. Mater. 26 (2014) 4947–4953. doi: 10.1002/adma.201400815. [DOI] [PubMed] [Google Scholar]
- [5].Elvira KS, Casadevall i Solvas X, Wootton RCR, de Mello AJ, The past, present and potential for microfluidic reactor technology in chemical synthesis., Nat. Chem 5 (2013) 905–915. doi: 10.1038/nchem.l753. [DOI] [PubMed] [Google Scholar]
- [6].Valencia PM, Farokhzad OC, Kamik R, Langer R, Microfluidic technologies for accelerating the clinical translation of nanoparticles, Nat. Nanotechnol. 7 (2012) 623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park J Il, Saffari A, Kumar S, Günther A, Kumacheva E, Microfluidic Synthesis of Polymer and Inorganic Particulate Materials, Annu. Rev. Mater. Res 40 (2010) 415–443. doi: 10.1146/annurev-matsci-070909-104514. [DOI] [Google Scholar]
- [8].Marre S, Jensen KF, Synthesis of micro and nanostructures in microfluidic systems., Chem. Soc. Rev. 39 (2010) 1183–1202. doi: 10.1039/b821324k. [DOI] [PubMed] [Google Scholar]
- [9].Hao N, Nie Y, Zhang JXJ, Microfluidic synthesis of functional inorganic micro-/nanoparticles and applications in biomedical engineering, Int. Mater. Rev. 63 (2018) 461–487. doi: 10.1080/09506608.2018.1434452. [DOI] [Google Scholar]
- [10].Haase MF, Stebe KJ, Lee D, Continuous Fabrication of Hierarchical and Asymmetric Bijel Microparticles, Fibers, and Membranes by Solvent Transfer-Induced Phase Separation ( STRIPS ), Adv. Mater. 27 (2015) 7065–7071. doi: 10.1002/adma.201503509. [DOI] [PubMed] [Google Scholar]
- [11].Uson L, Sebastian V, Arruebo M, Santamaria J, Continuous microfluidic synthesis and functionalization of gold nanorods, Chem. Eng. J. 285 (2016) 286–292. doi: 10.1016/j.cej.2015.09.103. [DOI] [Google Scholar]
- [12].Swain B, Hong MH, Kang L, Kim BS, Kim NH, Lee CG, Optimization of CdSe nanocrystals synthesis with a microfluidic reactor and development of combinatorial synthesis process for industrial production, Chem. Eng. J. 308 (2017) 311–321. doi: 10.1016/j.cej.2016.09.020. [DOI] [Google Scholar]
- [13].Di Carlo D, Inertial microfluidics., Lab Chip. 9 (2009) 3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- [14].Hao N, Nie Y, Shen T, Zhang JXJ, Microfluidics-enabled rational design of immunomagnetic nanomaterials and their shape effect on liquid biopsy, Lab Chip. 18 (2018) 1997–2002. doi: 10.1039/C8LC00273H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hao N, Nie Y, Zhang JXJ, Microfluidic Flow Synthesis of Functional Mesoporous Silica Nanofibers with Tunable Aspect Ratios, ACS Sustain. Chem. Eng. 6 (2018) 1522–1526. doi: 10.1021/acssuschemeng.7b03527. [DOI] [Google Scholar]
- [16].Hao N, Nie Y, Closson AB, Zhang JXJ, Microfluidic synthesis and on-chip enrichment application of two-dimensional hollow sandwich-like mesoporous silica nanosheet with water ripple-like surface, J. Colloid Interf. Sci. 539 (2019) 87–94. doi: 10.1016/j.jcis.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem. 57 (1985) 603–619. http://old.iupac.org/publications/pac/1985/pdf/5704x0603.pdf (accessed August 31, 2010). [Google Scholar]
- [18].Hao N, Chorsi HT, Zhang JXJ, Hierarchical Lotus Leaf-Like Mesoporous Silica Material with Unique Bilayer and Hollow Sandwich-Like Folds: Synthesis, Mechanism, and Applications, ACS Sustain. Chem. Eng. 5 (2017) 2044–2049. doi: 10.1021/acssuschemeng.6b02893. [DOI] [Google Scholar]
- [19].Shen D, Yang J, Li X, Zhou L, Zhang R, Li W, Chen L, Wang R, Zhang F, Zhao D, Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres, Nano Lett. 14 (2014) 923–932. doi: 10.1021/nl404316v. [DOI] [PubMed] [Google Scholar]
- [20].Nie Y, Hao N, Zhang JXJ, Ultrafast Synthesis of Multifunctional Submicrometer Hollow Silica Spheres in Microfluidic Spiral Channels, Sci. Rep. 7 (2017) 12616. doi: 10.1038/s41598-017-12856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hao NJ, Jayawardana KW, Chen X, Yan M, One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials, ACS Appl. Mater. Interfaces. 7 (2015) 1040–1045. doi: 10.1021/am508219g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kang YS, Risbud S, Rabolt JF, Stroeve P, Synthesis and Characterization of Nanometer-Size Fe3O4 and γ-Fe2O3 Particles, Chem. Mater. 8 (1996) 2209–2211. doi: 10.1021/cm960157j. [DOI] [Google Scholar]
- [23].Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG, (CdSe)ZnS Core-Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites, J. Phys. Chem. B. 101 (1997) 9463–9475. doi: 10.1021/jp971091y. [DOI] [Google Scholar]
- [24].Wei J, Wang H, Deng Y, Sun Z, Shi L, Tu B, Luqman M, Zhao D, Solvent evaporation induced aggregating assembly approach to three-dimensional ordered mesoporous silica with ultralarge accessible mesopores., J. Am. Chem. Soc. 133 (2011) 20369–20377. doi: 10.1021/ja207525e. [DOI] [PubMed] [Google Scholar]
- [25].Hao N, Li LF, Tang FQ, Facile preparation of ellipsoid-like MCM-41 with parallel channels along the short axis for drug delivery and assembly of Ag nanoparticles for catalysis, J. Mater. Chem. A. 2 (2014) 11565–11568. doi: 10.1039/C4TA01820F. [DOI] [Google Scholar]
- [26].Fang X, Liu Z, Hsieh MF, Chen M, Liu P, Chen C, Zheng N, Hollow mesoporous aluminosilica spheres with perpendicular pore channels as catalytic nanoreactors, ACS Nano. 6(2012) 4434–4444. doi: 10.1021/nn3011703. [DOI] [PubMed] [Google Scholar]
- [27].Squire PG, Moser P, O’Konski CT, The Hydrodynamic Properties of Bovine Serum Albumin Monomer and Dimer, Biochemistry. 7 (1968) 4261–4272. doi: 10.1021/bi00852a018. [DOI] [PubMed] [Google Scholar]
- [28].Wright AK, Thompson MR, Hydrodynamic structure of bovine serum albumin determined by transient electric birefringence, Biophys. J. 15 (1975) 137–141. doi: 10.1016/S0006-3495(75)85797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.