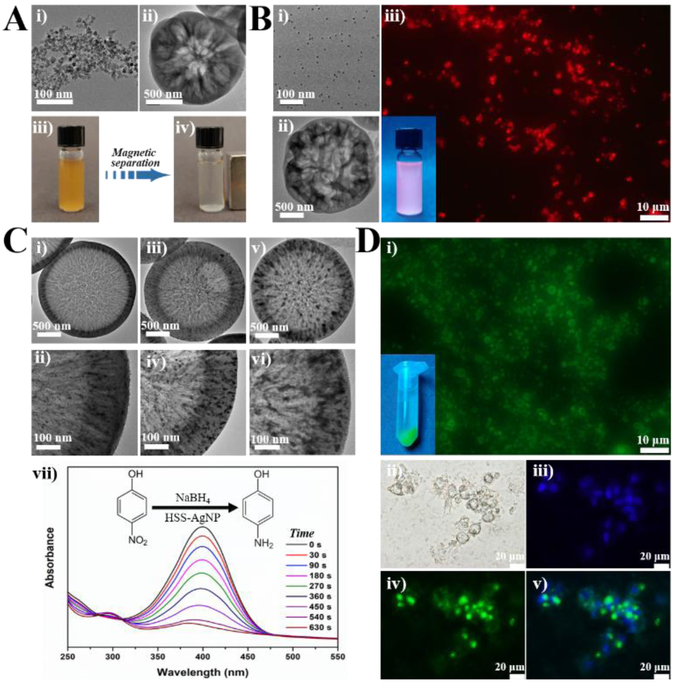
Figure 4.
Microfluidic synthesis of HSS-based functional materials. (A) TEM image of Fe3O4 MNP (i) used for the one-step synthesis of HSS-MNP (ii); (iii) magnetic separation behavior of HSS-MNP under an external magnet. (B) TEM image of CdSe@ZnS QD (i) used for the one-step synthesis of HSS-QD (ii); (iii) fluorescent image of HSS-QD under fluorescent microscope and the inset is a photo of HSS-QD solution under irradiation of an UV lamp. (C) TEM images of HSS-AgNP with different loading amounts of Ag (i, iii, and v) and their corresponding high magnification images (ii, iv, and vi); (vii) representative time-dependent UV-vis spectral changes of 4-nitrophenol catalyzed by the HSS-AgNP from (i). (D) Fluorescent images of HSS loaded with BSA-FITC (i) and the inset is a photo of HSS-BSA-FITC solution under irradiation of an UV lamp; (ii-iv) bright field and fluorescent images of MDA-MB-231 cells treated with HSS-BSA-FITC for 2 h, nuclei were counterstained with Hoechst 33342; a merged image is shown in (v).