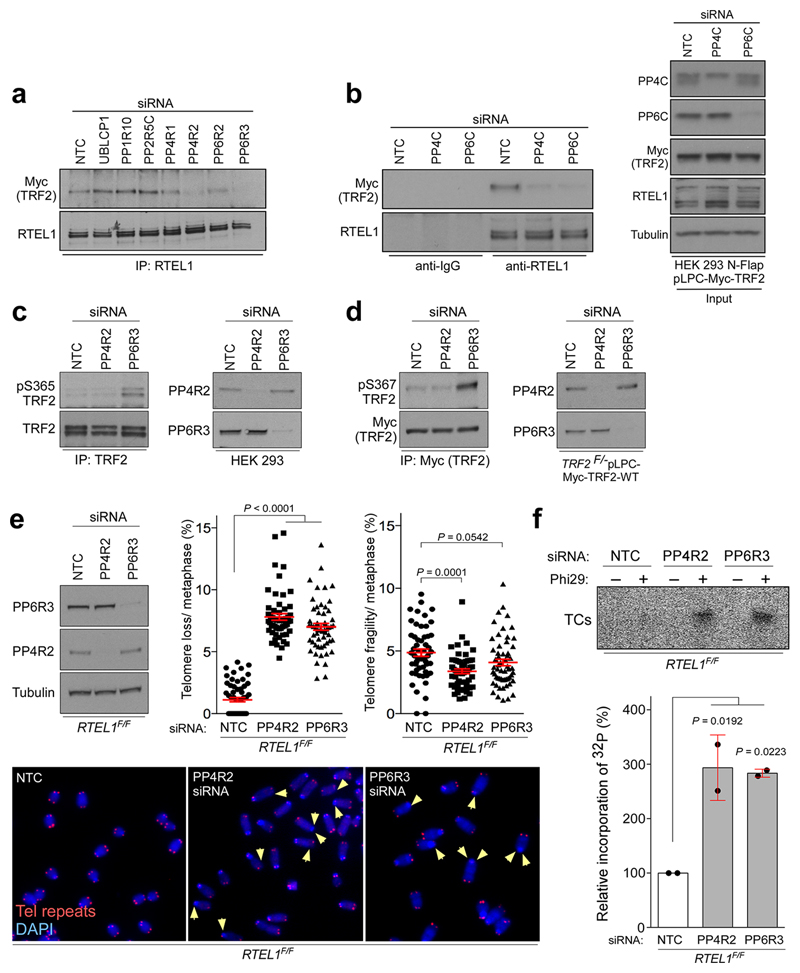
Extended Data Figure 5. Protein phosphatase 6 regulatory subunit 3 (PP6R3) controls phosphorylation of TRF2 at Ser365/367.
293 HEK cells expressing wt Myc-TRF2 were transfected with a non-targeting control (NTC) siRNA, or siRNAs against protein phosphatase regulatory subunits (a) or catalytic subunits (b). Cells were harvested, and whole-cell extracts were immunoprecipitated with anti-RTEL1 antibody. Immunocomplexes were resolved on SDS-PAGE and analysed by Western blotting as indicated. c, 293 HEK and (d) TRF2F/- MEFs expressing Myc-tagged wt TRF2 were subjected to PP4R2, PP6R3, or NTC siRNA. Whole-cell extracts were immunoprecipitated with anti-TRF2 antibody, and immunocomplexes resolved by SDS-PAGE and analysed for human (pS365 TRF2; left panel in c) or mouse phospho-TRF2 (pS367 TRF2; left panel in d). e, Frequency of telomere loss and telomere fragility per metaphase in RTEL1F/F MEFs transfected with NTC, PP4R2, or PP6R3 siRNA (one-way ANOVA, mean ± SEM, n=58 (NTC), n=57 (PP4R2), and n=55 (PP6R3) of analysed metaphases). Representative images of the telomere FISH experiments are shown on the bottom of (e). The arrowheads show loss of telomere signal. Red, telomere PNA FISH; blue, DAPI. Efficiency of siRNA was determined by Western blotting with PP6R3 and PP4R2 antibodies as indicated in upper left panel. f, Phi29-dependent telomere circles (TCs; upper panel) detected in the same cells as indicated in (e). The extent of [32P] incorporation was quantified (bottom panel) from the autoradiographs, and the level of [32P] incorporation by cells transfected with NTC was arbitrarily assigned a value of 100% (one-way ANOVA, mean ± SD, n= two independent experiments). In a - f the experiments were independently repeated at least two times with similar results.