FIG. 2.
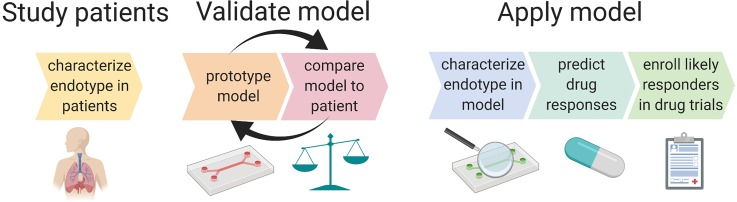
Iterative model design with validation against patient phenotype will lead to an endotype-specific model of ARDS that can be used for predictive enrichment of ARDS clinical trials. Endotype-specific metrics such as cytokine ratios, immune cell functions (e.g., bacterial killing, metabolism, and NETosis), and degree of surfactant production can be compared between patients and in vitro models. Iterative adjustments to model parameters, such as genetics (e.g., MUC5A upregulated epithelium), physical forces, degree of initial injury, degree of fibrosis, and the type and ratio of inflammatory mediators, could enable the development of a model that produces biomarkers or functional characteristics (e.g., response to therapeutics, response to mechanical strain, and immune cell phenotype changes such as enhanced NETosis), mimicking those of a specific endotype. The model should also be validated by testing functional outputs such as barrier function of the epithelium and tissue healing (scratch wound assay). The final model provides the opportunity for pathophysiological mechanisms of disease to be clarified and for drug candidates to be tested in vitro. Both pathophysiology and drug testing will help predict whether a certain endotype is likely to respond to novel treatments.