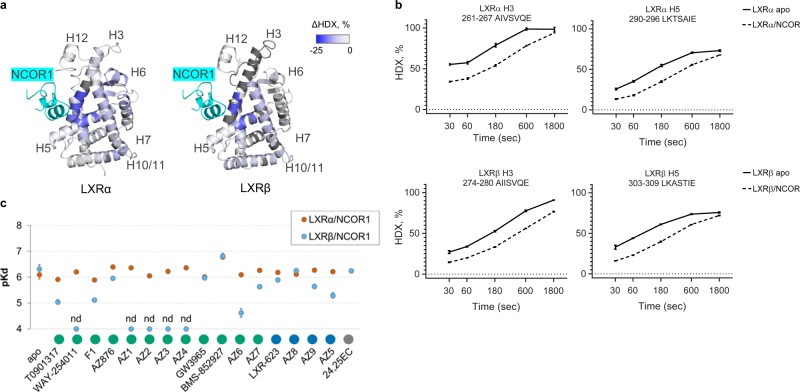
Fig. 5.
Functional implications of the LXR helices H3 and H5 in the cofactor recruitment. a Average differential HDX data mapped onto the LXRα (2ACL.pdb) and LXRβ (1PQC.pdb) crystal structures showing the difference in deuterium uptake between apo- and NCOR1-bound states. Full peptide list and statistical summary are presented in Supplementary Table 7. b Deuterium uptake plots for peptides from LXRα and LXRβ helices H3 and H5 in apo- and NCOR1-bound states. HDX-MS measurements were performed in triplicates; data are shown ± standard deviation. c Binding affinities of apo- and ligand-bound LXRα (orange circles) and LXRβ (cyan circles) to the immobilized NCOR1 corepressor peptide measured by SPR. Bars: ± standard error; nd: not detected. Original sensorgrams and derived equilibrium-binding affinity parameters are shown in Supplementary Fig. 20 (LXRα) and Supplementary Fig. 21 (LXRβ).