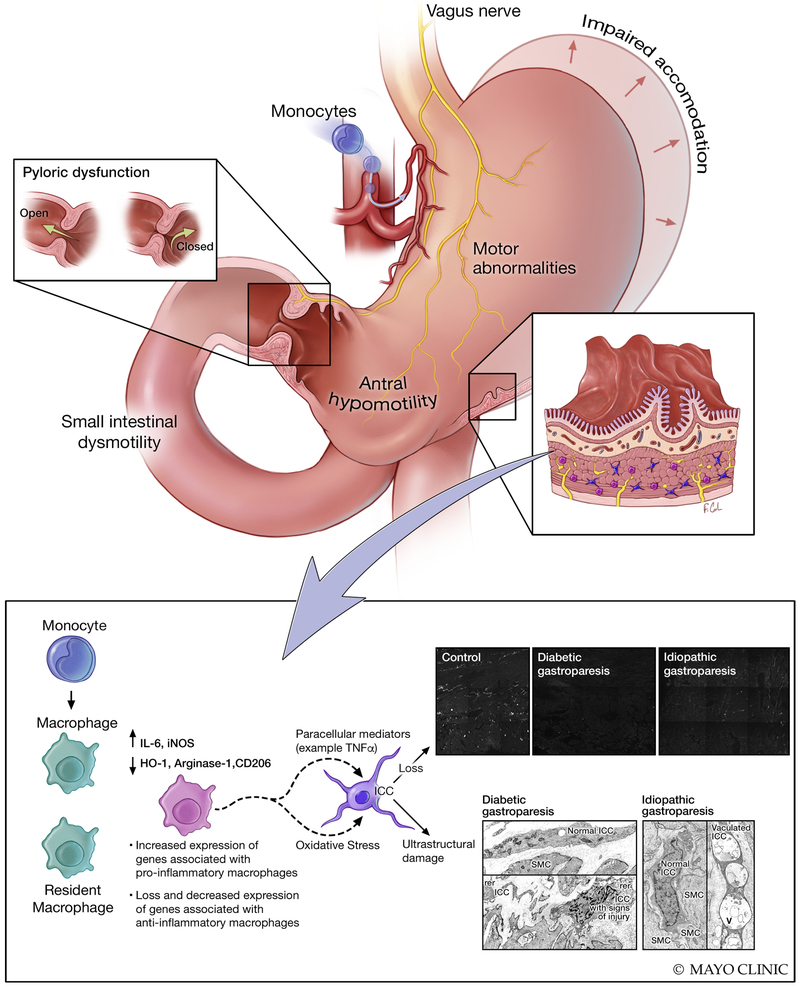
Figure 1: Gastrointestinal pathophysiological changes in human gastroparesis.
Physiological changes such as impaired accommodation of the gastric fundus/body, motor abnormalities including antral hypomotility, impaired pyloric relaxation and small intestinal dysmotility can result in impaired gastric emptying and clinical manifestations of gastroparesis. At a cellular level, loss of interstitial cells of Cajal (ICC) has been reported in over half of the gastroparesis patients and nearly all of the patients had signs of injury to ICC on ultrastructural studies. The loss of ICC correlated with delayed gastric emptying in diabetic gastroparesis. Ultrastructural studies also showed changes in nerves and smooth muscle cells which were less appreciable on immunohistochemistry. More recently, human studies have shown loss of macrophages with anti-inflammatory phenotype (CD206 positive, M2 or alternatively activated macrophages) and increased expression of genes associated with pro-inflammatory macrophages on transcriptomic analysis of full thickness biopsies. This is complemented by animal model studies of diabetic gastroparesis where an altered macrophage activation was shown to mediate injury to ICC likely through paracrine mediators. Additionally, CSF1op/op mice lacking macrophages were protected from development of gastroparesis in spite of having diabetes suggesting an essential role for immune cells in development of delayed gastric emptying. Immune mediated mechanisms likely play a critical role in pathogenesis of gastroparesis.