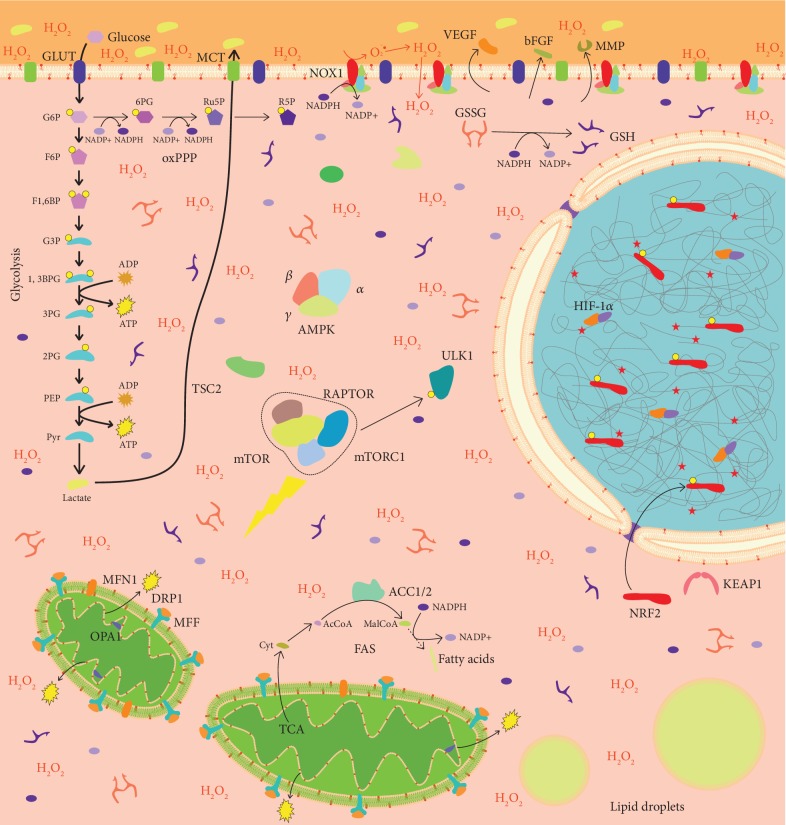
Figure 2.
LKB1 loss alters cancer cell biology. LBK1 loss and consequent lack of AMPK activation lead to mTORC1 assembly, resulting in autophagy inhibition. High metabolic requirements imposed by sustained proliferation are met through aerobic glycolysis (i.e., Warburg effect), driven by HIF-1α stabilization, which provides cancer cells with ATP and intermediates for anabolic reactions (not shown). Pyruvate is preferentially converted to lactate, which is excreted in the tumor microenvironment. Activation of ACC1 and ACC2 promotes fatty acid synthesis in the cytosol, by using citrate coming from mitochondria. NOX1 expression drives the assembly of NADPH oxidase complex, which produces ROS in the microenvironment. NOX-produced ROS enter the cell, thus inducing oxidative stress and activating NRF2 through the oxidation of KEAP1. Reduced expression of MFN1 and OPA1 and increased activity of DRP1, induced by HIF-1α activation, lead to mitochondrial fragmentation. Increased ROS levels and mitochondrial fission promote the secretion of proangiogenic factors in the microenvironment. Yellow circles: phosphate groups. Red phospholipids in membranes: peroxidised phospholipids. Red stars in the nucleus: DNA damage sites. G6P: glucose 6-phosphate; F6P: fructose 6-phosphate; F1,6BP: fructose 1,6-biphosphate; G3P: glyceraldehyde 3-phosphate; 1,3BPG: 1,3-biphosphoglycerate; 3PG: 3-phosphoglycerate; 2PG: 2-phosphoglycerate; PEP: phosphoenolpyruvate; Pyr: pyruvate; 6PG: 6-phosphogluconate; Ru5P: ribulose 5-phosphate; R5P: ribose 5-phosphate; AcCoA: acetyl-coA; MalCoA: malonyl-coA; Cit: citrate; GLUT: glucose transporter; MCT: monocarboxylate transporter; GSH: reduced glutathione; GSSG: oxidized glutathione; H2O2: hydrogen peroxide; oxPPP: oxidative pentose phosphate pathway; TCA: tricarboxylic acid cycle; FAS: fatty acid synthesis. mTORC1 targets are omitted. See the text for details.