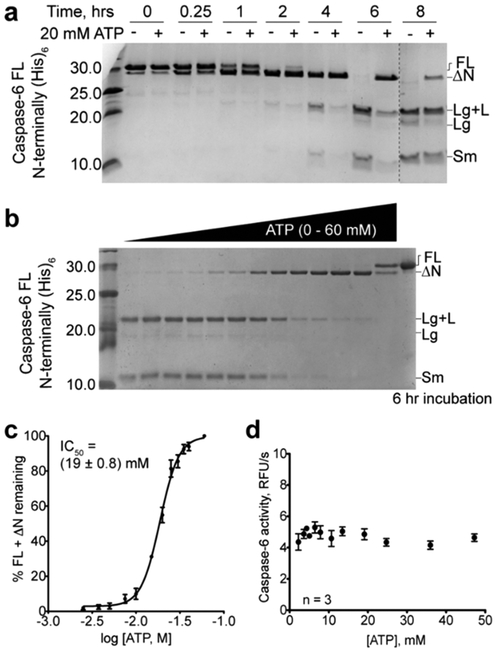
Figure 5.
ATP attenuates procaspase-6 autoactivation but not caspase-6 activity. (a) Self-proteolysis of procaspase-6 [caspase-6 FL N-terminally (His)6] in the absence and presence of 20 mM ATP at 37 °C for different time intervals (0, 0.25, 1, 2, 4, 6, and 8 h). An increasing incubation time promotes self-activation of procaspase-6, generating the large and small subunits of active caspase-6 (FL, full-length; ΔN, without the pro-domain; Lg+L, large subunit attached to the linker; Lg, large subunit; and Sm, small subunit). After 6 h, the pro-domain is fully cleaved in the absence of ATP but remains intact in the presence of ATP. (b) Self-proteolysis of procaspase-6 [FL N-terminally (His)6] after incubation for 6 h at 37 °C in the presence of various ATP concentrations (0, 2.5, 3.75, 5, 7.5, 10, 15, 20, 25, 30, 35, 40, and 60 mM). (c) Quantification of remaining band intensities to determine the potency for ATP-mediated inhibition of procaspase-6 self-activation (IC50 = 19 ± 0.8 mM). (d) Proteolytic activity of active caspase-6 measured using the fluorogenic VEID-AMC substrate in the presence of increasing concentrations of ATP.