Scheme 1.
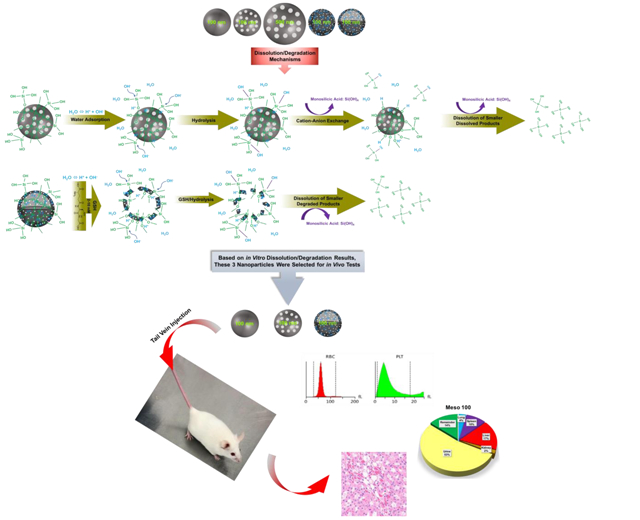
Schematic representation of in vitro and in vivo studies. The top panel illustrates the fabricated nanoparticles with variations in size, porosity, density, and composition (from left to right): Stöber 100, Meso 100, Meso 500, Disulfide Meso 100, and Disulfide Hollow 100 nanoparticles. The middle panel indicates degradation mechanisms. For regular SiO2 NPs, hydroxyl ions (OH−) attacking Si-O bonds are forming an unstable pentavalent structure which can be broken by attachment of other ions. In disulfide-based SiO2 NPs, a similar process is involved in addition to degradation via disulfide breakage in a redox environment containing GSH as a reducing agent which has high intracellular concentrations (2-10 mM) [33-35]. Release of monosilicic acid Si(OH)4 is the product of these processes. The bottom panel depicts the in vivo studies conducted in CD-1 mice. Three nanoparticles (Stöber 100, Meso 100, and Disulfide Hollow 100) were selected based on in vitro results. Particles were tested for in vivo degradation, toxicity, and biodistribution.
