Abstract
Background
The long-term safety of first-generation drug-eluting stent (DES) in acute coronary syndrome (ACS) was controversial.
Purpose
The purpose of this study was to establish 5-year real-world data regarding the long-term efficacy and safety of second-generation DES in Japanese patients with ACS.
Methods
The Tokyo-MD PCI study is a multicenter, observational cohort study enrolling consecutive patients who underwent everolimus-eluting stent (EES) implantation. The 5-year clinical events were compared between the ACS group (n = 644) and the stable coronary artery disease (SCAD) group (n = 1255). The primary efficacy endpoint was ischemia-driven target lesion revascularization (TLR), and the primary safety endpoint was the composite of all-cause death or myocardial infarction (MI).
Results
The median follow-up duration was 5.4 years. The cumulative incidence of ischemia-driven TLR was similar between ACS and SCAD (1 year: 3.0% versus 2.7%; P=0.682, 1–5 years: 2.7% versus 2.9%; P=0.864). The cumulative incidence of all-cause death or MI within 1 year was significantly higher in ACS than in SCAD (7.4% versus 3.8%; P < 0.001); however, ACS did not increase the risk of all-cause death or MI after adjusting confounders (adjusted hazard ratio, 1.260; 95% confidence interval, 0.774–2.053; P=0.352). From 1 to 5 years, the cumulative incidence of all-cause death or MI was not significantly different between ACS and SCAD (11.6% versus 11.4%; P=0.706). The cumulative incidence of very late stent thrombosis was low and similar between ACS and SCAD (0.2% versus 0.2%; P=0.942).
Conclusion
This real-world registry suggested that EES has comparable long-term efficacy and safety in patients with ACS and SCAD.
1. Introduction
The efficacy and safety of drug-eluting stent (DES) for acute coronary syndrome (ACS) patients have been continuously discussed since it was first introduced. Several early studies showed that first-generation DES has favorable short-term efficacy for reducing target lesion revascularization (TLR) in patients with acute myocardial infarction (AMI) compared with bare metal stent (BMS) [1, 2]. However, pathological examination of first-generation DES revealed that the vessel healing of the culprit lesions of AMI patients was delayed compared with those of stable coronary artery disease (SCAD) patients [3]. A large cohort study elucidated that ACS was an independent risk factor for late stent thrombosis (ST) after first-generation DES implantation [4]. Moreover, the GRACE study reported an increased late mortality after first-generation DES implantation in patients with AMI compared with BMS [5]. In addition, prolonged dual-antiplatelet therapy (DAPT) for AMI patients was associated with the increased risk of major bleeding [6, 7].
Everolimus-eluting stent (EES) was developed to advance the safety and efficacy of DES. Target lesion failure (TLF) and ST were consistently reduced with EES compared with first-generation DES [8–10]. Guidelines for the management of ST-segment elevation myocardial infarction (STEMI) indicated new-generation DES as the first-line coronary stent for reperfusion therapy [11, 12]. Recently, the 5-year results of the EXAMINATION study demonstrated the long-term benefits of EES in AMI patients compared with BMS [13].
In Japan, EES reduced TLF compared with sirolimus-eluting stent (SES) and there was no major concern about very late ST or late catch-up phenomenon [14, 15]. However, the long-term safety of EES for Japanese ACS patients, particularly beyond 3 years, has not been fully clarified. We conducted the Tokyo MD PCI study and registered complex Japanese patient populations for evaluation of the real-world data of EES [16]. In Japan, imaging device-guided EES implantation is performed at high rates. The imaging device-guided procedure allows optimal stenting and is expected to reduce late adverse events [17]. We expected that Japan would be suitable for a multicenter observational study for the long-term evaluation of EES. Therefore, we aimed to establish 5-year real-world data regarding the long-term efficacy and safety of EES for Japanese ACS patients in this study.
2. Methods
2.1. Study Design and Enrollment of Patients
The Tokyo-MD PCI study is a physician-initiated, multicenter, observational cohort study which was conducted to evaluate the real-world data of Japanese patients who underwent EES (Xience V®; Abbott Vascular, Santa Clara, CA, USA; Promus®; Boston Scientific, Marlborough, MA, USA) implantation. Previously, we published the details and 4-year results of the Tokyo-MD PCI study [16]. From January 2010 to December 2011, consecutive 1918 patients were enrolled from 22 hospitals in Japan. The patients were divided into the ACS group or the SCAD group based on the clinical manifestation at the first procedure. The ACS group was further stratified into STEMI, non-ST elevation myocardial infarction (NSTEMI), and unstable angina pectoris (UAP) according to the Third Universal Definition of Myocardial Infarction [18]. Myocardial infarction (MI) was diagnosed when cardiac biomarker values rise above the 99th percentile upper reference limit with the following: symptom of ischemia, new significant ST-T change or new left bundle branch block, development of pathological Q wave, imaging evidence of new regional wall motion abnormality, and identification of an intracoronary thrombus by angiography. MI accompanied with ST-segment elevation in two contiguous leads was defined as STEMI; in contrast, absence of ST-segment elevation at presentation was diagnosed as NSTEMI. Patients without elevated biomarker values were diagnosed as unstable angina.
The clinical information on patient and lesion characteristics at baseline, procedures of EES implantation, and follow-up data were retrospectively collected from medical records. Follow-up included a clinical visit or telephone contact. The median follow-up period for surviving patients was 5.4 years (interquartile range 4.3–6.1 years). Clinical follow-up was completed in 93.4% of surviving patients at 1 year, 82.3% at 3 years, and 70.7% at 5 years. Follow-up angiography within 1 year was left to the discretion of each hospital.
This study was approved by the institutional ethical review board at Tokyo Medical and Dental University and according to the Ethical Guidelines for Epidemiological Research. We published all relevant details of this study instead of obtaining informed consent.
2.2. Stent Implantation and Antiplatelet Therapy
The procedures of EES implantation and medical therapy, including the duration of DAPT, were left to the discretion of each attending physician. At the index procedure, heparin was used for anticoagulation. The recommended antiplatelet therapy was aspirin (81 mg/day) and thienopyridine (200 mg/day ticlopidine or 75 mg/day clopidogrel). Persistent discontinuation of DAPT was defined as withdrawal lasting 2 months.
2.3. Study Endpoints
The primary efficacy endpoint was ischemia-driven TLR, and the primary safety endpoint was the composite of all-cause death or MI. The secondary endpoint included cardiac death, target vessel MI, TLF (composite of cardiac death, target vessel MI, and ischemia-driven TLR), and definite and probable ST. The landmark analysis of patients who did not have the primary and secondary events at 1 year was performed. All-cause death and MI were judged by the investigator at each center. Cardiac death and MI were defined according to standardized definitions [19]. TLR was defined as either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) because of restenosis or thrombosis of the target lesion, including lesions within 5 mm of the stent borders. TLR was defined as ischemia-driven if the procedure was associated with a positive functional study result or ischemic symptoms. ST was defined according to the Academic Research Consortium definition. Major bleeding event was defined as type 3 or 5 bleeding according to the Bleeding Academic Research Consortium (BARC) criteria.
2.4. Statistical Analysis
Categorical variables were compared using the chi-square test. Continuous variables are presented as the mean and standard deviation, and categorical variables are presented as numbers and percentages. Continuous variables were compared using Student's t test and Dunnett's test based on their distribution. The cumulative incidence of clinical endpoint was estimated by using the Kaplan–Meier method and compared with the log-rank test. Hazard ratio (HR) was calculated in the univariate and multivariate Cox proportional hazards models. In the univariate models, the HR for ACS and 33 confounders that were reported as being important factors in previous studies were calculated. A multivariate Cox proportional hazard model was used to adjust the differences in baseline characteristics. In the multivariable analysis, we incorporated variables with P values <0.05 in the univariate models. The results are expressed as adjusted HR and their 95% confidence intervals (CI). All analyses were performed using SPSS version 10 (IBM in Armonk, Cary, NY, USA).
3. Results
3.1. Patient Enrollment
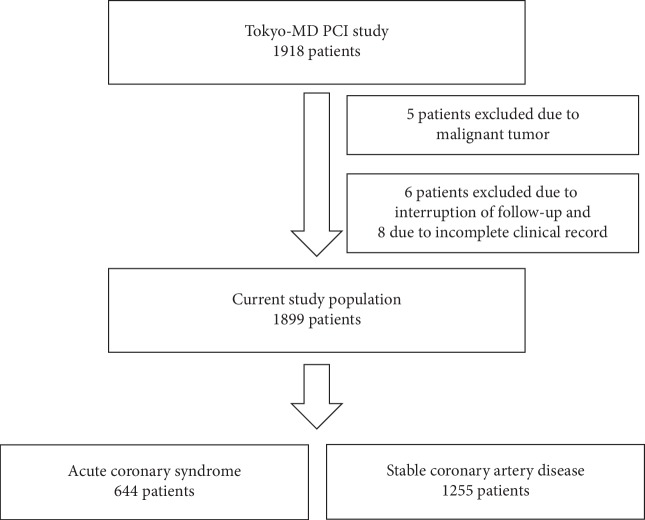
Figure 1 shows the flowchart of the Tokyo-MD PCI study. Five patients with a malignant tumor, 6 patients who dropped out of medical follow-up, and 8 patients whose clinical records were incomplete were excluded. After the exclusion of the 19 patients, 1899 patients were evaluated. Studied patients were divided into the ACS group (n = 644) or the SCAD group (n = 1255).
Figure 1.
Flowchart of the Tokyo-MD PCI study.
3.2. Baseline Characteristics
Patient and lesion characteristics are shown in Table 1. The ACS patients had higher prevalence of chronic kidney disease without hemodialysis, left ventricular ejection fraction <35%, cardiogenic shock, triple-vessel disease, left anterior descending coronary artery lesion, and ostial lesion and were aged more than 80 years compared with SCAD patients. In contrast, the prevalence of diabetes mellitus, hemodialysis, peripheral artery disease, previous PCI, previous CABG, previous MI, restenotic lesion, and chronic total occlusion were higher in SCAD patients. The ACS group included patients with cardiogenic shock status (5.1%). Intra-aortic balloon pumping and percutaneous cardiopulmonary support were used in 40 (6.2%) and 10 (1.6%) patients of the ACS group. Beta-blocker was more frequently prescribed in ACS patients than in SCAD patients. An imaging device was used in 94% of the studied procedures. Follow-up coronary artery angiography within 1 year was performed in 61% of the patients.
Table 1.
Patient and lesion characteristics.
| Variable | ACS group | SCAD group | P value |
|---|---|---|---|
| Patients (n) | 644 | 1255 | |
| Age (years) | 70.5 ± 9.9 | 69.6 ± 9.9 | 0.050 |
| Age ≥80 years | 135 (21.0%) | 195 (15.5%) | 0.003 |
| Male | 461 (71.6%) | 945 (75.3%) | 0.080 |
| ST-segment elevation myocardial infarction | 218 (33.9%) | 0 | <0.001 |
| Non-ST-segment elevation myocardial infarction | 190 (29.5%) | 0 | <0.001 |
| Unstable angina pectoris | 236 (36.6%) | 0 | <0.001 |
| Stable angina pectoris | 0 | 885 (70.1%) | <0.001 |
| Silent myocardial ischemia | 0 | 370 (29.5%) | <0.001 |
| Current smoker | 139 (20.7%) | 233 (18.6%) | 0.275 |
| Hypertension | 469 (72.8%) | 922 (73.5%) | 0.765 |
| Dyslipidemia | 533 (82.8%) | 1025 (81.7%) | 0.558 |
| Diabetes mellitus | 244 (37.9%) | 544 (43.3%) | 0.022 |
| Chronic kidney disease | |||
| Without hemodialysis | 129 (20.0%) | 200 (15.9%) | 0.027 |
| With hemodialysis | 24 (3.7%) | 102 (8.1%) | <0.001 |
| Cardiogenic shock state at procedure | 33 (5.1%) | 0 | <0.001 |
| Intra-aortic balloon pumping | 40 (6.2%) | 3 (0.2) | <0.001 |
| Percutaneous cardiopulmonary support | 10 (1.6%) | 0 (0.0) | <0.001 |
| Left ventricular ejection fraction <35% | 48 (7.5%) | 57 (4.5%) | 0.009 |
| Triple vessel disease | 106 (16.5%) | 155 (12.4%) | 0.014 |
| Peripheral artery disease | 50 (7.8%) | 140 (11.2%) | 0.020 |
| History of stroke | 59 (9.2%) | 129 (10.3%) | 0.440 |
| History of myocardial infarction | 156 (24.2%) | 419 (33.4%) | <0.001 |
| Previous percutaneous coronary intervention | 172 (26.7%) | 509 (40.6%) | <0.001 |
| Previous coronary artery bypass grafting | 30 (4.7%) | 89 (7.1%) | 0.038 |
| Medication | |||
| Use of aspirin | 641 (99.5%) | 1251 (99.7%) | 0.617 |
| Use of thienopyridine | 636 (98.8%) | 1229 (97.9%) | 0.197 |
| Use of cilostazol | 20 (3.1%) | 45 (3.6%) | 0.586 |
| Use of anticoagulant agent | 69 (10.7%) | 143 (11.4%) | 0.656 |
| Use of statin | 509 (79.0%) | 985 (78.5%) | 0.781 |
| Use of beta-blocker | 348 (54.0%) | 562 (44.8%) | <0.001 |
| Use of angiotensin-converting enzyme inhibitor | 107 (16.6%) | 173 (13.8%) | 0.100 |
| Use of angiotensin receptor blocker | 282 (43.8%) | 566 (45.1%) | 0.586 |
| Use of proton pump inhibitor | 387 (60.1%) | 752 (59.9%) | 0.942 |
| Coronary lesion location | <0.001 | ||
| Left anterior descending coronary artery | 311 (48.3%) | 519 (41.4%) | |
| Left circumflex coronary artery | 100 (15.5%) | 246 (19.6%) | |
| Right coronary artery | 184 (28.6%) | 435 (34.7%) | |
| Left main coronary artery | 47 (7.3%) | 51 (4.1%) | |
| Bypass graft | 2 (0.3%) | 4 (0.3%) | |
| Type B2/C lesion | 509 (79.0%) | 982 (78.2%) | 0.691 |
| Restenotic lesion | 56 (8.7%) | 151 (12.0%) | 0.027 |
| Chronic total occlusion | 24 (3.7%) | 121 (9.6%) | <0.001 |
| Ostial lesion | 104 (16.1%) | 169 (13.5%) | 0.115 |
| Bifurcation | 160 (24.8%) | 301 (24.0%) | 0.679 |
| Severe calcification on angiography | 130 (20.2%) | 275 (21.9%) | 0.385 |
| Number of stents used (n) | 1.4 ± 0.7 | 1.4 ± 0.7 | 0.742 |
| Average stent diameter (mm) | 2.98 ± 0.36 | 2.97 ± 0.36 | 0.626 |
| Use of 2.5 mm diameter stent | 212 (32.9%) | 439 (35.0%) | 0.370 |
| Total stent length (mm) | 29.5 ± 17.4 | 29.1 ± 17.4 | 0.737 |
| Total stent length ≥28 mm | 193 (30.0%) | 375 (29.9%) | 0.968 |
| Use of imaging device for stent placement | 565 (87.7%) | 1224 (97.5%) | <0.001 |
| Initial thrombolysis in myocardial infarction grade flow | <0.001 | ||
| 0 | 131 (20.3%) | 121 (9.6%) | |
| 1 | 49 (1.6%) | 9 (0.7%) | |
| 2 | 105 (16.3%) | 12 (0.9%) | |
| 3 | 359 (55.7%) | 1113 (88.7%) | |
| Final thrombolysis in myocardial infarction grade flow | 0.149 | ||
| 0 | 1 (0.2%) | 0 | |
| 1 | 5 (0.8%) | 3 (0.2%) | |
| 2 | 10 (1.6%) | 15 (1.2%) | |
| 3 | 628 (97.6%) | 1237 (98.6%) | |
Note. Data are given as n (%) or as the mean ± standard deviation. ACS: acute coronary syndrome; SCAD: stable coronary artery disease.
3.3. DAPT Discontinuation
Figure 2 shows the cumulative incidence of persistent discontinuation of DAPT. DAPT was discontinued in 7.9% at 1 year and in 45.7% at 5 years in the ACS group and in 9.0% at 1 year and in 44.6% at 5 years in the SCAD group. There was no significant difference in persistent discontinuation of DAPT at 5 years between the two groups (P=0.405).
Figure 2.
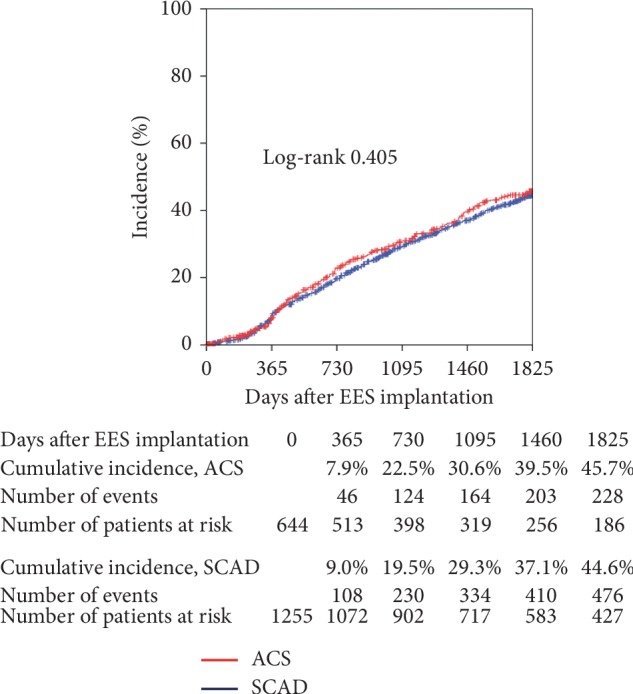
Cumulative incidence of persistent discontinuation of dual-antiplatelet therapy. Persistent discontinuation was defined as withdrawal lasting for at least 2 months. EES: everolimus-eluting stent; ACS: acute coronary syndrome; SCAD: stable coronary artery disease.
3.4. Clinical Outcome
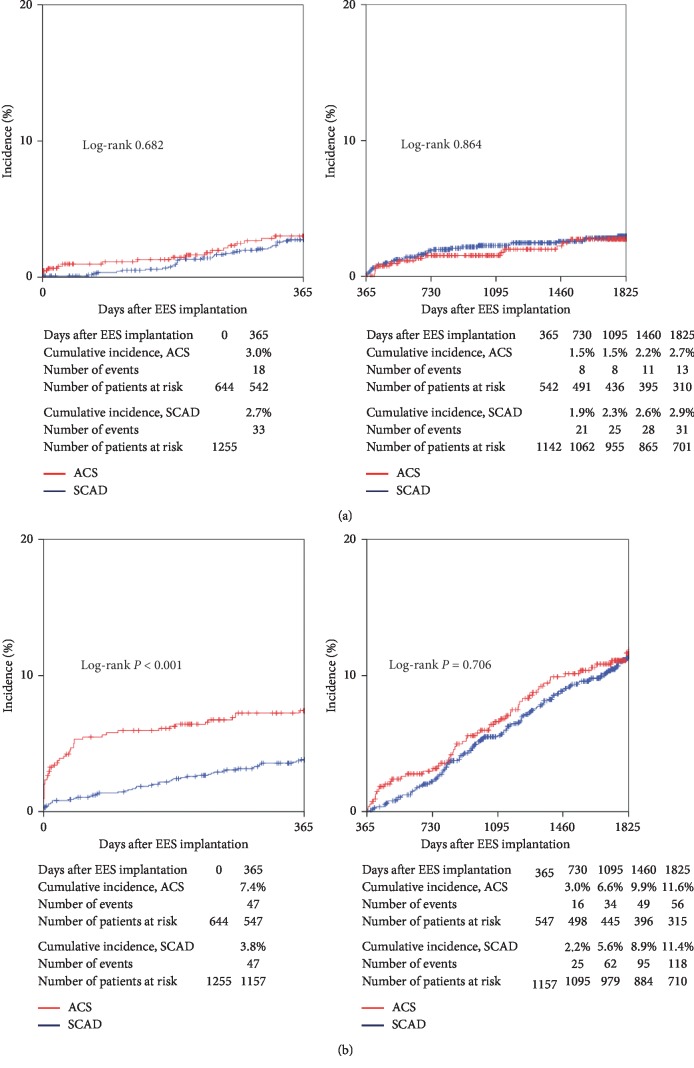
Table 2 shows the clinical events in the ACS and SCAD groups. Figure 3(a) shows the cumulative incidence of the primary efficacy endpoint (ischemia-driven TLR). The cumulative incidence of ischemia-driven TLR was similar between the ACS and SCAD groups at 5 years (1 year: 3.0% versus 2.7%; P=0.682; from 1 to 5 years: 2.7% versus 2.9%; P=0.864).
Table 2.
Clinical events through 5 years.
| At 1 year | From 1 to 5 years | |||||
|---|---|---|---|---|---|---|
| ACS | SCAD | P value | ACS | SCAD | P value | |
| All-cause death | 35 (5.5%) | 30 (2.4%) | <0.001 | 44 (9.1%) | 105 (10.0%) | 0.460 |
| Cardiac death | 25 (3.9%) | 15 (1.2%) | <0.001 | 18 (3.7%) | 32 (3.1%) | 0.373 |
| Myocardial infarction | 15 (2.4%) | 18 (1.5%) | 0.132 | 16 (3.3%) | 17 (1.8%) | 0.008 |
| Target vessel myocardial infarction | 11 (1.8%) | 9 (0.7%) | 0.039 | 7 (1.4%) | 10 (1.0%) | 0.227 |
| All-cause death or myocardial infarction | 47 (7.4%) | 47 (3.8%) | <0.001 | 56 (11.6%) | 118 (11.4%) | 0.706 |
| Ischemia-driven TLR | 18 (3.0%) | 33 (2.7%) | 0.682 | 13 (2.7%) | 31 (2.9%) | 0.864 |
| Target lesion failure | 47 (7.5%) | 53 (4.3%) | 0.003 | 32 (6.8%) | 64 (6.2%) | 0.514 |
| BARC bleeding | ||||||
| Type 3 | 8 (1.3%) | 12 (1.0%) | 0.498 | 7 (1.6%) | 26 (2.6%) | 0.085 |
| Type 5 | 0 (0.0%) | 3 (0.2%) | 0.229 | 2 (0.4%) | 3 (0.3%) | 0.705 |
| Stent thrombosis | ||||||
| Definite | 8 (1.3%) | 4 (0.3%) | 0.015 | 1 (0.2%) | 2 (0.2%) | 0.942 |
| Probable | 2 (0.3%) | 1 (0.1%) | 0.223 | 0 (0%) | 0 (0%) | NA |
| Definite or probable | 10 (1.6%) | 5 (0.4%) | 0.006 | 1 (0.2%) | 2 (0.2%) | 0.942 |
| Possible | 2 (0.3%) | 5 (0.4%) | 0.814 | 15 (1.5%) | 8 (1.6%) | 0.974 |
Note. Incidences of events were calculated by the Kaplan–Meyer method. Cumulative incidences were compared with the log-rank test. ACS: acute coronary syndrome; SCAD: stable coronary artery disease; TLR: target lesion revascularization; BARC: bleeding academic research consortium.
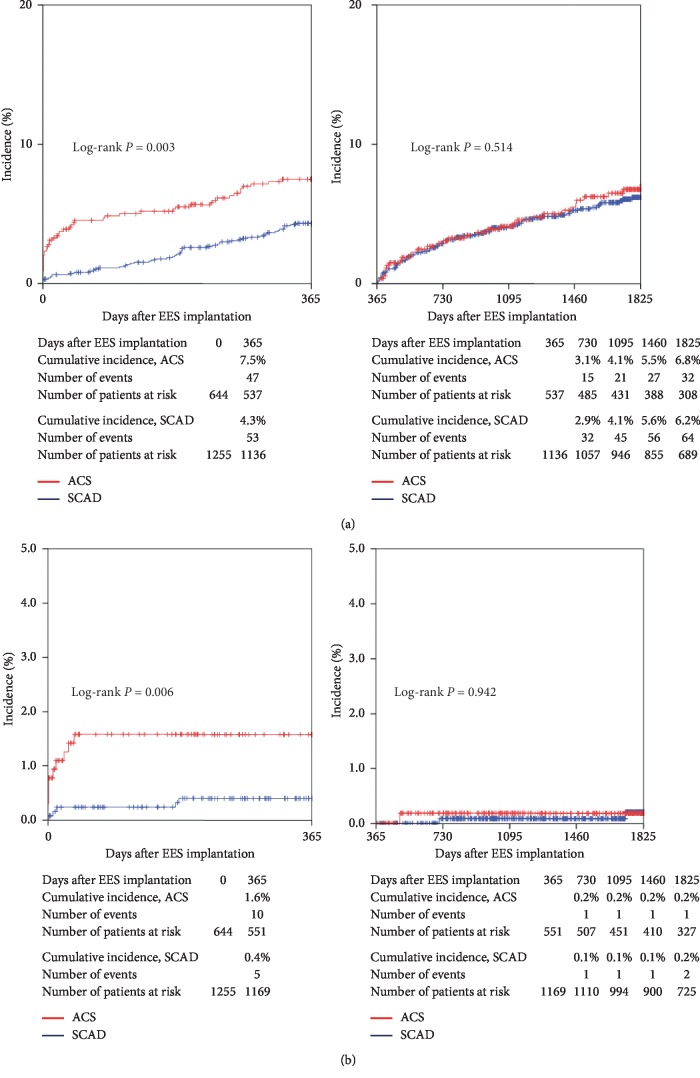
Figure 3.
(a) Cumulative incidence of the primary efficacy endpoint (ischemia-driven target lesion revascularization). (b) Cumulative incidence of the primary safety endpoint (the composite of all-cause death or myocardial infarction). EES: everolimus-eluting stent; ACS: acute coronary syndrome; SCAD: stable coronary artery disease.
The cumulative incidence of the safety endpoint (the composite of all-cause death or MI) is shown in Figure 3(b). The cumulative incidence of all-cause death or MI at 1 year was significantly higher in the ACS group than in the SCAD group (7.4% versus 3.8%; P < 0.001). The higher risk of the safety endpoint in the ACS group at 1 year was mainly driven by the increase in all-cause death compared with the SCAD group (5.5% versus 2.4%; P < 0.001). From 1 to 5 years, the cumulative incidence of all-cause death or MI was not significantly different between the ACS and SCAD groups (11.6% versus 11.4%; P=0.706).
Figure 4(a) shows the cumulative incidence of TLF. TLF occurred significantly more frequently in the ACS group than in the SCAD group at 1 year (7.5% versus 4.3%; P=0.003). From 1 to 5 years, the cumulative incidence of TLF was not significantly different between the ACS and SCAD groups (6.8% versus 6.2%; P=0.514).
Figure 4.
(a) Cumulative incidence of target lesion failure. (b) Cumulative incidence of stent thrombosis. EES: everolimus-eluting stent; ACS: acute coronary syndrome; SCAD: stable coronary artery disease.
Figure 4(b) shows the cumulative incidence of definite and probable ST. ST within 1 year was observed significantly more frequent in the ACS group than in the SCAD group (1.6% versus 0.4%; P=0.006). However, the incidence of ST from 1 to 5 years was very low in both groups, and there was no significant difference between the ACS and SCAD groups (0.2% versus 0.2%; P=0.942).
3.5. Univariate and Multivariate Analysis of the Primary Safety End-point
We performed the univariate and multivariate analysis to calculate the adjusted HR of the primary safety endpoint (Table 3 and 4) because there was significantly increased risk in the safety endpoint in the ACS group at 1 year.
Table 3.
Univariate and multivariate analysis for the safety endpoint (the composite of all-cause death or myocardial infarction) at 1 year.
| Univariate Cox proportional hazard model | Multivariate Cox proportional hazard model | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| ACS versus SCAD | 2.033 (1.357–3.046) | 0.001 | 1.260 (0.774–2.053) | 0.352 |
| Age ≥80 years | 2.369 (1.536–3.656) | <0.001 | 2.145 (1.358–3.386) | 0.001 |
| Male | 0.732 (0.475–1.130) | 0.159 | ||
| Current smoker | 1.260 (0.782–2.032) | 0.342 | ||
| Hypertension | 1.201 (0.745–1.937) | 0.451 | ||
| Dyslipidemia | 0.459 (0.298–0.708) | <0.001 | 0.993 (0.486–2.029) | 0.985 |
| Diabetes mellitus | 1.291 (0.861–1.935) | 0.216 | ||
| Chronic kidney disease without hemodialysis | 1.484 (0.921–2.393) | 0.105 | ||
| Chronic kidney disease with hemodialysis | 4.658 (2.927–7.499) | <0.001 | 3.895 (2.124–7.143) | <0.001 |
| Left ventricular ejection fraction <35% | 8.352 (5.366–13.002) | <0.001 | 3.223 (1.913–5.431) | <0.001 |
| Peripheral artery disease | 2.009 (1.118–3.396) | 0.009 | 1.261 (0.692–2.299) | 0.449 |
| History of stroke | 1.608 (0.911–2.837) | 0.101 | ||
| History of myocardial infarction | 0.923 (0.590–1.442) | 0.724 | ||
| Previous percutaneous coronary intervention | 0.825 (0.535–1.273) | 0.384 | ||
| Previous coronary artery bypass grafting | 1.403 (0.680–2.895) | 0.360 | ||
| Triple vessel disease | 2.470 (1.527–3.881) | <0.001 | 1.380 (0.844–2.255) | 0.199 |
| Cardiogenic shock status at procedure | 28.050 (16.864–46.656) | <0.001 | 14.228 (6.511–31.090) | <0.001 |
| Left anterior descending coronary artery | 0.835 (0.552–1.263) | 0.392 | ||
| Left circumflex coronary artery | 0.584 (0.311–1.095) | 0.094 | ||
| Right coronary artery | 1.345 (0.889–2.034) | 0.161 | ||
| Left main coronary artery | 2.066 (1.039–4.108) | 0.038 | 1.360 (0.560–1.807) | 0.360 |
| Type B2/C lesion | 1.677 (0.933–3.011) | 0.084 | ||
| Restenotic lesion | 0.546 (0.239–1.247) | 0.151 | ||
| Chronic total occlusion | 1.119 (0.542–2.309) | 0.761 | ||
| Ostial lesion | 2.921 (1.893–4.506) | <0.001 | 2.605 (1.600–4.239) | <0.001 |
| Bifurcation | 1.349 (0.867–2.099) | 0.185 | ||
| Severe calcification on angiography | 2.725 (1.808–4.107) | <0.001 | 1.364 (0.845–2.203) | 0.204 |
| Use of 2.5 mm diameter stent | 1.378 (0.914–2.077) | 0.126 | ||
| Total stent length ≥28 mm | 1.479 (0.976–2.242) | 0.065 | ||
| Use of imaging device for stent placement | 0.281 (0.164–0.481) | <0.001 | 0.765 (0.378–1.547) | 0.456 |
| Use of anticoagulant agent | 0.937 (0.499–1.757) | 0.838 | ||
| Use of statin | 0.429 (0.283–0.652) | <0.001 | 0.853 (0.419–1.737) | 0.662 |
| Use of beta-blocker | 0.752 (0.499–1.133) | 0.173 | ||
| Use of angiotensin-converting enzyme inhibitor | 0.625 (0.408–0.957) | 0.031 | 0.684 (0.436–1.073) | 0.098 |
| Use of angiotensin receptor blocker | 1.176 (0.687–2.014) | 0.554 | ||
Note. ACS: acute coronary syndrome; SCAD: stable coronary artery disease; HR: hazard ratio; CI: confidence interval.
Table 4.
Univariate and multivariate analysis for the safety endpoint (the composite of all-cause death or myocardial infarction) from 1 to 5 years.
| Univariate Cox proportional hazard model | Multivariate Cox proportional hazard model | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| ACS versus SCAD | 1.058 (0.789–1.418) | 0.706 | ||
| Age ≥80 years | 2.192 (1.606–2.992) | <0.001 | 2.079 (1.511–2.860) | <0.001 |
| Male | 1.099 (0.793–1.522) | 0.571 | ||
| Current smoker | 0.735 (0.501–1.077) | 0.114 | ||
| Hypertension | 1.283 (0.922–1.786) | 0.140 | ||
| Dyslipidemia | 0.615 (0.447–0.846) | 0.003 | 0.749 (0.456–1.231) | 0.254 |
| Diabetes mellitus | 1.189 (0.903–1.564) | 0.217 | ||
| Chronic kidney disease without hemodialysis | 1.931 (1.412–2.641) | <0.001 | 1.747 (1.244–2.454) | 0.001 |
| Chronic kidney disease with hemodialysis | 3.966 (2.731–5.759) | <0.001 | 3.000 (1.931–4.661) | <0.001 |
| Left ventricular ejection fraction <35% | 2.931 (1.866–4.604) | <0.001 | 2.158 (1.345–3.464) | <0.001 |
| Peripheral artery disease | 2.663 (1.897–3.739) | <0.001 | 1.992 (1.384–2.868) | <0.001 |
| History of stroke | 1.645 (1.110–2.473) | 0.013 | 1.221 (0.815–1.831) | 0.333 |
| History of myocardial infarction | 1.307 (0.982–1.739) | 0.067 | ||
| Previous percutaneous coronary intervention | 1.150 (0.869–1.521) | 0.329 | ||
| Previous coronary artery bypass grafting | 1.595 (1.005–2.530) | 0.047 | 0.921 (0.567–1.497) | 0.741 |
| Triple vessel disease | 1.625 (1.146–2.303) | 0.006 | 1.252 (0.873–1.797) | 0.222 |
| Cardiogenic shock status at procedure | 4.032 (1.287–12.625) | 0.017 | 2.984 (0.909–9.792) | 0.071 |
| Left anterior descending coronary artery | 0.742 (0.559–0.985) | 0.039 | 0.777 (0.580–1.041) | 0.091 |
| Left circumflex coronary artery | 1.175 (0.839–1.645) | 0.347 | ||
| Right coronary artery | 1.175 (0.883–1.562) | 0.268 | ||
| Left main coronary artery | 1.221 (0.665–2.242) | 0.519 | ||
| Type B2/C lesion | 1.622 (1.112–2.365) | 0.012 | 1.444 (0.983–2.121) | 0.061 |
| Restenotic lesion | 1.095 (0.715–1.676) | 0.677 | ||
| Chronic total occlusion | 0.742 (0.423–1.301) | 0.297 | ||
| Ostial lesion | 1.139 (0.772–1.678) | 0.510 | ||
| Bifurcation | 0.952 (0.688–1.319) | 0.769 | ||
| Severe calcification on angiography | 1.888 (1.401–2.544) | <0.001 | 1.183 (0.852–1.642) | 0.315 |
| Use of 2.5 mm diameter stent | 0.865 (0.644–1.163) | 0.337 | ||
| Total stent length ≥28 mm | 1.096 (0.812–1.478) | 0.550 | ||
| Use of imaging device for stent placement | 1.064 (0.564–2.009) | 0.848 | ||
| Use of anticoagulant agent | 1.843 (1.309–2.595) | <0.001 | 1.428 (1.003–2.035) | 0.048 |
| Use of statin | 0.636 (0.467–0.866) | 0.004 | 0.946 (0.578–1.549) | 0.826 |
| Use of beta-blocker | 1.038 (0.789–1.365) | 0.791 | ||
| Use of angiotensin-converting enzyme inhibitor | 0.903 (0.685–1.190) | 0.470 | ||
| Use of angiotensin receptor blocker | 1.084 (0.739–1.588) | 0.681 | ||
Note. ACS: acute coronary syndrome; SCAD: stable coronary artery disease; HR: hazard ratio; CI: confidence interval.
At 1 year, the univariate analysis showed that the ACS group had higher HR of the safety endpoint compared with the SCAD group (HR, 2.033; 95% CI, 1.357–3.046; P=0.001). The multivariate analysis revealed that age older than 80 years, hemodialysis, left ventricular ejection fraction <35%, cardiogenic shock, and ostial lesion had higher adjusted HR of the safety endpoint at 1 year. However, the adjusted HR of the ACS group at 1 year was not significantly higher compared with the SCAD group (adjusted HR, 1.260; 95% CI, 0.774–2.053; P=0.352).
From 1 to 5 years, the ACS group did not increase the HR of the safety endpoint compared with the SCAD group (HR, 1.058; 95% CI, 0.706–1.418; P=0.70). By the multivariate analysis, age older than 80 years, chronic kidney disease without hemodialysis, hemodialysis, left ventricular ejection fraction <35%, peripheral artery disease, and anticoagulation therapy were determined as independent predictors of the safety endpoint from 1 to 5 years.
4. Discussion
In the present study, we have for the first time compared the 5-year real-world outcome after EES implantation between ACS and SCAD patients in Japan. The main findings are as follows. (1) The incidence of efficacy endpoint was similar between the two groups at 5 years. (2) The incidence of safety endpoint and TLF within 1 year was significantly higher in the ACS group compared with the SCAD group. However, those were not different beyond 1 year. The multivariate analysis clarified that ACS did not increase the adjusted HR of the safety endpoint throughout 5 years. (3) The cumulative incidence of ST beyond 1 year was very low and similar between ACS and SCAD groups.
ACS has common pathological backgrounds, including disruption or erosion of the atherosclerotic plaques, alterations in circulating prothrombotic or antifibrinolytic mediators, and acute coronary thrombogenicity [20]. Although ACS treatment has notably developed, STEMI still has approximately 5 to 6% in-hospital mortality and 7 to 18% 1-year mortality rates [11].
Previously, the long-term outcome of Japanese ACS patients treated with first-generation DES was reported in the largest SES study (j-Cypher) and in the single-center registry of Kyoto University Hospital (which used SES in 82% of the patients) [21, 22]. Although the Tokyo-MD PCI study was retrospective, we performed consecutive patient registrations from multicenter hospitals and there were small numbers of excluded patients.
The Tokyo-MD PCI study had several remarkable differences compared with previous overseas studies of EES on the following points. (1) Compared with the XIENCE V USA study (an all-comer observational registry in the United States) [23], the Tokyo-MD PCI study registered more complex patient populations, such as those undergoing hemodialysis, with cardiogenic shock status, with low ejection fraction, with left main disease, and with angiographic heavy calcification. (2) The rate of imaging device-guided EES implantation was high. The imaging device-guided procedure is expected to reduce late adverse events. These features of this study distinguished the Tokyo-MD PCI study from previous EES studies. Therefore, we think this registry has the new information about the clinical practice of EES implantation.
4.1. Problems of First-Generation DES Implantation in ACS Patients
First-generation DES has the problem of late catch-up phenomenon and late ST [24, 25]. Clinical researches examining the safety of first-generation DES in ACS patients showed conflicting results about the risk of death or ST [5, 26–29]. The pathology of first-generation DES revealed the presence of delayed arterial healing [30], and incomplete endothelial coverage was the histological predictor of ST [31]. Regarding the culprit lesions of ACS, underlying plaque morphology including large necrotic core, ruptured fibrous cap, and thrombus attributed to the further delayed arterial response to first-generation DES [3]. Optical coherence tomography (OCT) also validated the further delayed vascular healing in patients with UAP after SES implantation compared with SCAD [32]. This distinctive response to first-generation DES between ACS and SCAD lesions implies that lesion morphology plays an important role in vascular healing. Moreover, heterogeneous healing responses remained after 5 years of first-generation DES implantation [33].
4.2. Improved Vascular Response of EES Compared with First-Generation DES
EES consists of a thin strut platform (81 μm), coated with 7.8-μm-thick durable fluorinated copolymer and 1.0 μg/mm everolimus [34]. In human autopsy analysis, EES showed favorable strut coverage with less inflammation and fibrin deposition compared with SES and paclitaxel-eluting stent (PES) [35]. Sawada compared the arterial healings in STEMI patients at 7 months between EES and SES using OCT and angioscopy [36]. OCT showed that frequencies of uncovered and malapposed struts in EES were lower than those in SES in STEMI patients. Angioscopic analysis also presented more homogenous neointimal coverage and less intrastent thrombus in EES than those in SES. These improved pathological findings support the greater clinical safety of EES compared with first-generation DES in ACS patients.
4.3. Efficacy Endpoint after EES Implantation in ACS Patients
In the Tokyo-MD PCI study, EES had comparable efficacy in reducing ischemia-driven TLR between the ACS and SCAD groups at 5 years. Similar result was previously observed in the j-Cypher study, in which the incidence of TLR after SES implantation was not different between the ACS and non-ACS patients at 3 years [21]. In the XIENCE V USA study, AMI was not associated with the increased risk of TLR [23]. Our data conformed to the existing data showing that ACS did not increase the risk of TLR after DES implantation.
4.4. Safety Endpoint after EES Implantation in ACS Patients
In the Tokyo-MD PCI study, the ACS group had the higher risk of the safety endpoint at 1 year than in the SCAD group. However, multivariate analysis revealed that ACS was not an independent predictor of the safety endpoint at 1 year. This study demonstrated that severity and comorbidity of ACS patients, such as hemodialysis, low ejection fraction, and cardiogenic shock status, were mainly attributed to the risk of the safety endpoint at 1 year.
In contrast, the incidence of the safety endpoint from 1 to 5 years was similar between the ACS and SCAD groups despite the differences in patient background. Although the incidence of MI was more frequent in the ACS group than in the SCAD group from 1 to 5 years, the number of MI events was small in both groups, resulting in no statistical difference in the safety endpoint. In addition, the low incidence of very late ST in both groups might contribute to the favorable long-term safety after EES implantation.
4.5. ST after EES Implantation in Patients with ACS
The cumulative incidence of ST at 1 year was higher in the ACS group than in the SCAD group in the Tokyo-MD PCI study, and most incidence of ST occurred within 1 month. These results were explained because stenting for the culprit lesions of ACS has higher risk of acute and subacute ST due to the instability of the atheromatous plaque and the presence of thrombus [37].
The incidence of ST beyond 1 year was low and not different between the two groups. In the era of first-generation DES, subanalysis of the j-Cypher study showed that ACS patients tend to have higher risk of ST beyond 1 year than non-ACS patients, and ST continued to occur up to 3 years in ACS patients [21]. In the Kyoto University registry, definite ST occurred in 3.0% of ACS patients at 5 years after DES implantation in Japan [22].
EES reduced the risk of very late ST in ACS patients compared with first-generation DES in the subgroup analysis of a prospective cohort study [38]. In addition, the Tokyo-MD PCI study had similar incidence of definite or probable ST at 5 years compared with the EXAMINATION study (Tokyo-MD PCI 1.8% versus EXAMINATION 2.0%) [13]. Our data were consistent with those of previous overseas studies demonstrating the benefit of EES in reducing the risk of late ST in ACS patients.
However, the incidence of ST in the Tokyo-MD PCI study was too low to analyze the differences between the two groups and to decide the predictors of ST by the multivariate analysis. The evolution of new-generation DES substantially reduced late and very late ST; therefore, a larger-scale clinical study is required for more detailed statistical analysis.
4.6. Unsolved Problems of EES
Another pathological problem of DES was lipid-rich neoatherosclerosis, which might lead to subsequent ST from the disruption of neointimal hyperplasia. A previous study reported the frequencies of neoatherosclerosis were similar between EES and SES [36]. It is uncertain whether ACS increases the risk of neoatherosclerosis after EES implantation.
The appropriate DAPT duration after DES implantation has not been established yet. The guideline on the duration of DAPT recommended 6 months DAPT for SCAD and at least 12 months DAPT for ACS [39]. However, the duration of DAPT in this study was longer than that of the current guideline in both groups. In the early period of EES implantation, physicians preferred the longer DAPT duration for the concern of the late ST. Recently, several clinical studies have tried to shorten the duration of DAPT [40]. Further studies are needed to examine whether the duration of DAPT affects clinical outcome in ACS patients.
The rate of imaging device use was high in the Tokyo-MD PCI study. The univariate analysis showed that the use of imaging device reduced the risk of safety endpoint within 1 year compared with the nonuse of imaging device. The recent study showed that intravascular ultrasound-guided primary PCI for STEMI was not associated with a lower risk for target-vessel revascularization or ST [41]. The efficacy of imaging devices in DES implantation for ACS patients should be investigated further.
4.7. Study Limitation
This study has several limitations. First, this registry was a nonrandomized, observational cohort study. There was a possibility that unknown confounding factors had influence on the clinical events. Second, clinical information was collected retrospectively. Although the median follow-up period exceeded 5 years, under-reporting of the clinical events was possible. Third, the procedure of EES implantation and the duration of DAPT were left to the discretion of each attending physician. Detailed information on procedures, such as thrombus aspiration, distal protection, and direct stenting was not available. Consequently, this study did not allow the analysis of the effects of the procedure and the medical therapy in the occurrences of the clinical endpoints. Forth, this study is the subanalysis of the Tokyo-MD PCI study and we do not have sample size estimation. Fifth, the number of studied patients was small for the statistical analysis of the risk of ST. Further studies are needed to investigate the risk of very late ST in ACS patients.
5. Conclusion
This real-world registry suggested that EES has comparable long-term efficacy and safety in Japanese patients with ACS and SCAD at 5-year follow-up. These findings might have a great impact on determining the strategy of revascularization therapy for ACS patients.
Acknowledgments
The authors are thankful to the participating centers and investigators (Supplementary File 1).
Data Availability
The data used to support the findings of this study are restricted by the ethical review board at Tokyo Medical and Dental University in order to protect patient's privacy. Data are available from Shunji Yoshikawa for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors have no conflicts of interest with regard to this study.
Supplementary Materials
Participating centers and investigators.
References
- 1.Laarman G. J., Suttorp M. J., Dirksen M. T., et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. New England Journal of Medicine. 2006;355(11):1105–1113. doi: 10.1056/nejmoa062598. [DOI] [PubMed] [Google Scholar]
- 2.Spaulding C., Henry P., Teiger E., et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. New England Journal of Medicine. 2006;355(11):1093–1104. doi: 10.1056/nejmoa062006. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa G., Finn A. V., Joner M., et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients. Circulation. 2008;118(11):1138–1145. doi: 10.1161/circulationaha.107.762047. [DOI] [PubMed] [Google Scholar]
- 4.Wenaweser P., Daemen J., Zwahlen M., et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. Journal of the American College of Cardiology. 2008;52(14):1134–1140. doi: 10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Steg P. G., Fox K. A., Eagle K. A., et al. Mortality following placement of drug-eluting and bare-metal stents for ST-segment elevation acute myocardial infarction in the Global Registry of Acute Coronary Events. European Heart Journal. 2009;30(3):321–329. doi: 10.1093/eurheartj/ehn604. [DOI] [PubMed] [Google Scholar]
- 6.Buresly K., Eisenberg M. J., Zhang X., Pilote L. Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction. Archives of Internal Medicine. 2005;165(7):784–789. doi: 10.1001/archinte.165.7.784. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T., Hiasa Y., Hosokawa S., et al. The potential benefits and risks of the use of dual antiplatelet therapy beyond 6 months following sirolimus-eluting stent implantation for low-risk patients. Journal of Cardiology. 2011;57(3):283–289. doi: 10.1016/j.jjcc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Palmerini T., Biondi-Zoccai G., Riva D. D., et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. The Lancet. 2012;379(9824):1393–1402. doi: 10.1016/s0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 9.Gada H., Kirtane A. J., Newman W., et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents. JACC: Cardiovascular Interventions. 2013;6(12):1263–1266. doi: 10.1016/j.jcin.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Jensen L. O., Thayssen P., Christiansen E. H., et al. Safety and efficacy of everolimus- versus sirolimus-eluting stents. Journal of the American College of Cardiology. 2016;67(7):751–762. doi: 10.1016/j.jacc.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 11.O’Gara P. T., Kushner F. G., Ascheim D. D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 12.Windecker S., Kolh P., Alfonso F., et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the european society of cardiology (ESC) and the european association for cardio-thoracic surgery (EACTS) developed with the special contribution of the european association of percutaneous cardiovascular interventions (EAPCI) European Heart Journal. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 13.Sabaté M., Brugaletta S., Cequier A., et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. The Lancet. 2016;387(10016):357–366. doi: 10.1016/s0140-6736(15)00548-6. [DOI] [PubMed] [Google Scholar]
- 14.Shiomi H., Kozuma K., Morimoto T., et al. Long-term clinical outcomes after everolimus- and sirolimus-eluting coronary stent implantation. Circulation: Cardiovascular Interventions. 2014;7(3):343–354. doi: 10.1161/circinterventions.113.001322. [DOI] [PubMed] [Google Scholar]
- 15.Aoki J., Kozuma K., Awata M., et al. Three-year clinical outcomes of everolimus-eluting stents from the post-marketing surveillance study of cobalt-chromium everolimus-eluting stent (XIENCE V/PROMUS) in Japan. Circulation Journal. 2016;80(4):906–912. doi: 10.1253/circj.cj-15-1181. [DOI] [PubMed] [Google Scholar]
- 16.Kurihara K., Ashikaga T., Sasaoka T., Yoshikawa S., Isobe M. Incidence and predictors of early and late target lesion revascularization after everolimus-eluting stent implantation in unselected patients in Japan. Catheterization and Cardiovascular Interventions. 2017;90(1):78–86. doi: 10.1002/ccd.26964. [DOI] [PubMed] [Google Scholar]
- 17.Medical Advisory Secretariat. Intravascular ultrasound to guide percutaneous coronary interventions: an evidence-based analysis. Ontario Health Technology Assessment Series. 2006;6(12):1–97. [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K., Alpert J. S., Jaffe A. S., et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/cir.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 19.Cutlip D. E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials. Circulation. 2007;115(17):2344–2351. doi: 10.1161/circulationaha.106.685313. [DOI] [PubMed] [Google Scholar]
- 20.Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/circulationaha.105.537878. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi R., Kimura T., Morimoto T., et al. Safety and efficacy of sirolimus-eluting stent implantation in patients with acute coronary syndrome in the real world. The American Journal of Cardiology. 2010;106(11):1550–1560. doi: 10.1016/j.amjcard.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Kawaji T., Shiomi H., Morimoto T., et al. Long-term efficacy and safety outcomes after unrestricted use of drug-eluting stents in patients with acute coronary syndrome. Circulation Journal. 2014;78(7):1628–1635. doi: 10.1253/circj.cj-13-1388. [DOI] [PubMed] [Google Scholar]
- 23.Naidu S. S., Krucoff M. W., Rutledge D. R., et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation. JACC: Cardiovascular Interventions. 2012;5(6):626–635. doi: 10.1016/j.jcin.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T., Morimoto T., Nakagawa Y., et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation. Circulation. 2012;125(4):584–591. doi: 10.1161/circulationaha.111.046599. [DOI] [PubMed] [Google Scholar]
- 25.Galløe A. M., Kelbæk H., Thuesen L., et al. 10-year clinical outcome after randomization to treatment by Sirolimus- or paclitaxel-eluting Coronary Stents. Journal of the American College of Cardiology. 2017;69(6):616–624. doi: 10.1016/j.jacc.2016.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Mauri L., Silbaugh T. S., Garg P., et al. Drug-eluting or bare-metal stents for acute myocardial infarction. New England Journal of Medicine. 2008;359(13):1330–1342. doi: 10.1056/nejmoa0801485. [DOI] [PubMed] [Google Scholar]
- 27.Brar S. S., Leon M. B., Stone G. W., et al. Use of drug-eluting stents in acute myocardial infarction. Journal of the American College of Cardiology. 2009;53(18):1677–1689. doi: 10.1016/j.jacc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 28.De Luca G., Valgimigli M., Spaulding C., et al. Short and long-term benefits of sirolimus-eluting stent in ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. Journal of Thrombosis and Thrombolysis. 2009;28(2):200–210. doi: 10.1007/s11239-009-0305-7. [DOI] [PubMed] [Google Scholar]
- 29.Spaulding C., Teiger E., Commeau P., et al. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty) JACC: Cardiovascular Interventions. 2011;4(1):14–23. doi: 10.1016/j.jcin.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Joner M., Finn A. V., Farb A., et al. Pathology of drug-eluting stents in humans. Journal of the American College of Cardiology. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Finn A. V., Joner M., Nakazawa G., et al. Pathological correlates of late drug-eluting stent thrombosis. Circulation. 2007;115(18):2435–2441. doi: 10.1161/circulationaha.107.693739. [DOI] [PubMed] [Google Scholar]
- 32.Kubo T., Imanishi T., Kitabata H., et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable Angina pectoris. JACC: Cardiovascular Imaging. 2008;1(4):475–484. doi: 10.1016/j.jcmg.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Räber L., Zanchin T., Baumgartner S., et al. Differential healing response attributed to culprit lesions of patients with acute coronary syndromes and stable coronary artery after implantation of drug-eluting stents: an optical coherence tomography study. International Journal of Cardiology. 2014;173(2):259–267. doi: 10.1016/j.ijcard.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Kukreja N., Onuma Y., Serruys P. W. Xience V™ everolimus-eluting coronary stent. Expert Review of Medical Devices. 2009;6(3):219–229. doi: 10.1586/erd.09.1. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka F., Vorpahl M., Nakano M., et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129(2):211–223. doi: 10.1161/circulationaha.113.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawada T., Shinke T., Otake H., et al. Comparisons of detailed arterial healing response at seven months following implantation of an everolimus- or sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction. International Journal of Cardiology. 2013;168(2):960–966. doi: 10.1016/j.ijcard.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Karrillon G. T. J., Morice M. C., Benveniste E., et al. Intracoronary stent implantation without ultrasound guidance and with replacement of conventional anticoagulation by antiplatelet therapy. Circulation. 1996;94(7):1519–1527. doi: 10.1161/01.cir.94.7.1519. [DOI] [PubMed] [Google Scholar]
- 38.Räber L., Magro M., Stefanini G. G., et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents. Circulation. 2012;125(9):1110–1121. doi: 10.1161/circulationaha.111.058560. [DOI] [PubMed] [Google Scholar]
- 39.Levine G. N., Bates E. R., Bittl J. A., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123–e155. doi: 10.1161/cir.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 40.Natsuaki M., Morimoto T., Yamamoto E., et al. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: ShortT and optimal duration of dual antiplatelet therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial. Cardiovascular Intervention and Therapeutics. 2016;31(3):196–209. doi: 10.1007/s12928-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakatsuma K., Shiomi H., Morimoto T., et al. Intravascular ultrasound guidance vs. angiographic guidance in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction - long-term clinical outcomes from the CREDO-kyoto AMI registry. Circulation Journal. 2016;80(2):477–484. doi: 10.1253/circj.cj-15-0870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Participating centers and investigators.
Data Availability Statement
The data used to support the findings of this study are restricted by the ethical review board at Tokyo Medical and Dental University in order to protect patient's privacy. Data are available from Shunji Yoshikawa for researchers who meet the criteria for access to confidential data.