Figure 2.
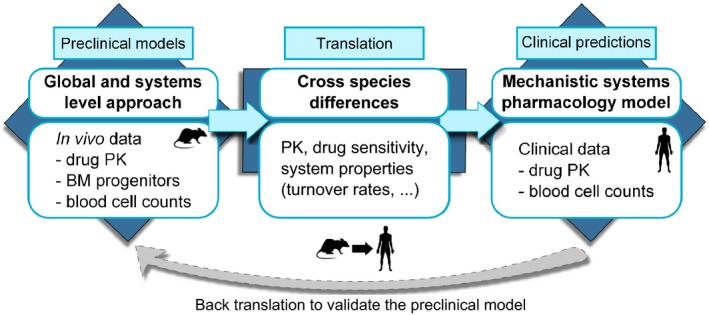
Graphical representation of our theoretical approach developed to investigate drug‐induced haematological toxicities. The first step consisted of defining a novel global and quantitative system pharmacology model able to integrate data from different sources (i.e., rat carboplatin PK, bone marrow effects, and peripheral blood counts) and describe carboplatin‐induced myelosuppression profiles in rats. Then, we considered the cross‐species differences between rat and human to update model parameter values. Last, we generated clinical predictions. In addition, when clinical data are available, back‐translation can also be performed (dotted gray arrow). BM, bone marrow; PK, pharmacokinetic.
