FIGURE 3. Homology models of (a) TSP-1-like domains and (b) F-spondin-like domains.
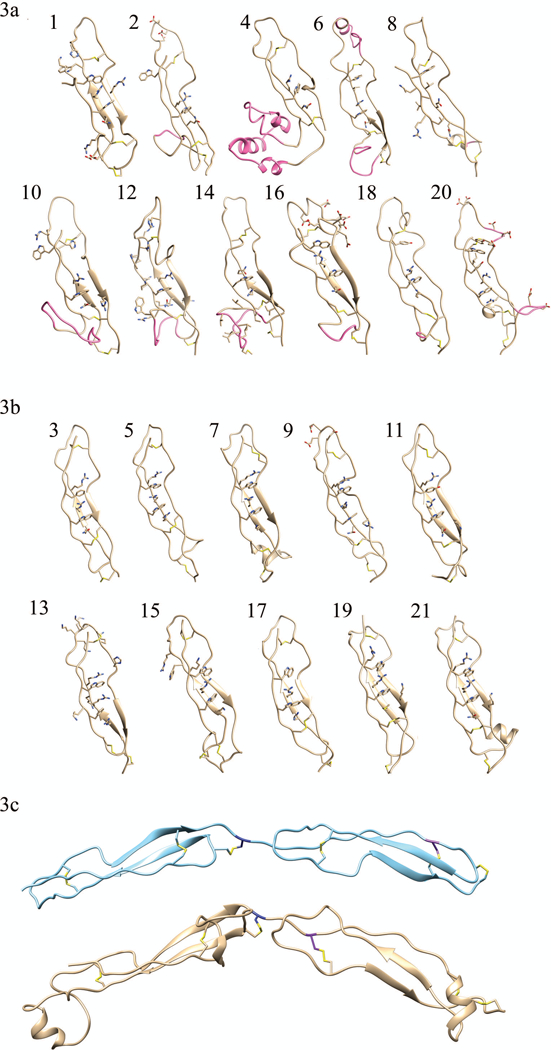
Individual domain homology models of the eleven TSP-1-like domains (1, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20) and the ten F-spondin-like domains (3, 5, 7, 9, 11, 13, 15, 17, 19, 21). In each domain the residues participating in disulfide bonds and those forming the Trp-ladder are shown. Insertions into the canonical fold (AB loop insertion, the jar handle insertion) of the TSP-1-like domains are shown in pink. Domain 8 has a relatively small BC loop insertion (shown in pink) and no Trp-ladder forming cation-pi. (3c) Disulfide bonding pattern switch is shown. The consecutive second and third TSP-1 domains of thrombospondin are shown in light blue ribbon structure, (pdb ID: 1LSL). TSP-1-like domain (domain 6) followed by a F-spondin-like domain (domain 7) in THSD7A are shown beneath the thrombospondin structure in gold. The C6 Cys is colored in navy blue of the first TSR repeat. It can be seen that the C1 Cys residue (purple) of the following tandem repeat shifts from a late position in thrombospondin to an early position in THSD7A. This alternating late then early C1 Cys results in the TSP-1 like followed by F-spondin like pattern of THSD7A.
