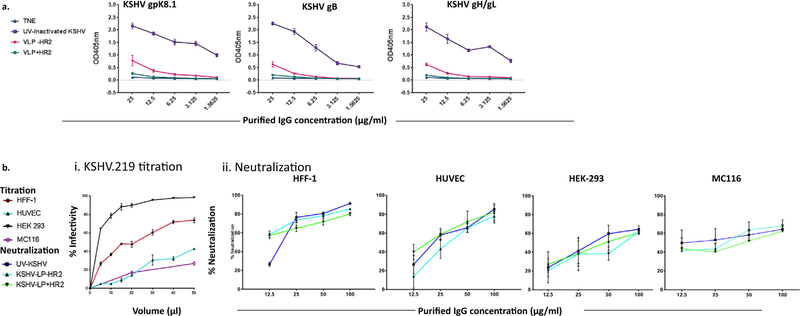
Fig. 4.
In vitro neutralization capabilities of purified IgGs from rabbits immunized with KSHV-LPs+/−HR2 in diverse cell types. (a) Titration of purified IgGs specific to KSHV glycoproteins by ELISA. Purification of IgG antibodies was conducted by pooling equal amounts of Day 49 sera from immunized rabbits (n = 6) from each of the four treatment/control, groups. KSHV-glycoprotein IgG-specific antibody titer was determined using ELISA with recombinant tagged proteins gpK8.1, gB, and gH/gL as targets; results of quadruplicate replicates are expressed as mean ± SD. (b) rKSHV-eGFP.219 titration and neutralization assays in epithelial, fibroblast, endothelial, and B cells. Panel i. rKSHV-eGFP.219 virus titration in diverse cell types. Individual cell lines were seeded overnight at a density of 5 × 105 in quadruplicate in 48-well-plates. The individual cell lines were then incubated with 5, 10, 20, 30, or 50 ml of the purified virus in a total volume of 100 µl of virus plus serum-free media for 24 h at 37°C. Infected cells (eGFP+) were quantified using FACS by acquiring a total of 10,000 events. Panel ii. Neutralization activity in HEK-293 cells, HFF-1 cells, HUVEC, and MC116 B cell lines. Neutralization activities were determined by incubating rKSHV-eGFP.219 virus and purified IgGs of varying concentrations (12.5, 25, 50, or 100 µg/ml) from all groups of test animals for 1 h at 37 °C, before being added to previously seeded cells in a 48-well plate. After 1 h incubation at 37 °C, the cells were thoroughly washed three times with 1 × PBS before adding growth media. The level of neutralization was determined in the cells after 24 h using FACS to quantify the number of eGFP+cells. Cells incubated with virus or with media alone served as positive and negative controls, respectively. Results of quadruplicate replicates are expressed as mean ± SD.