Abstract
Background:
Research has demonstrated associations between hormonal fluctuations during the menstrual cycle and women’s alcohol use. This association has been explained by mood changes that, for some women, accompany decreasing levels of progesterone during the menstrual cycle, particularly during the late luteal/premenstrual phase. The current study examined whether participants’ daily ratings of mood interact with changing levels of progesterone to predict alcohol use.
Method:
Young adult women attended two sessions scheduled two weeks apart, during which they completed questionnaires and provided salivary samples for the assay of progesterone levels. In the intervening two weeks, participants completed daily logs of their mood, alcohol use, and menses. Ordered Generalized Linear Mixed Models assessed the effects of daily mood (examined as both a within- and between-subject variable) on the likelihood of drinking, as a function of menstrual cycle phase and changes in progesterone across the two weeks.
Results:
One standard deviation increase in progesterone corresponded to a 1.61 decrease in the odds of drinking. This main effect was moderated by daily mood. Women were more likely to drink during a decrease in progesterone on days they rated their mood as negative, whereas during an increase in progesterone they were more likely to drink on days they reported a positive mood. Between-subject analyses showed that women who reported lower overall mood during the two-week period were more likely to drink with an increase in progesterone and less likely with a decrease.
Conclusions:
Women’s likelihood to drink increased when they experienced negative mood in the context of decreasing levels of progesterone, whereas the negative-mood/drinking association was mitigated among those with increasing levels of progesterone. However, compared to women who on average had an overall more positive mood, women with an overall lower mood (and corresponding higher levels of depression and anxiety at baseline) did not experience the protective effects of rising progesterone levels on drinking.
Keywords: Women, Alcohol, Progesterone, Menstrual Cycle, Mood
1. Introduction
Data from the most recent National Epidemiologic Survey on Alcohol and Related Conditions (NESARC III) show alarming increases in alcohol misuse among women. Since 2000, the rates of high-risk drinking in women has increased by 57.9% and rates of alcohol use disorder (AUD) have increased by 83.7% (Grant et al., 2017). Alcohol misuse has also increased among young adults, with 12-month rates of high risk drinking (4 or more standard drinks on any day for women, 5 or more for men) among 18- to 29-year-old men and women at 19.3% (Grant et al., 2017). It remains unclear why women misuse alcohol, despite protective factors historically afforded by their gender [including gender roles, coping style, and personality traits (Nolen-Hoeksema, 2004; Tuchman, 2010)]. Understanding factors that contribute to alcohol misuse among young adult women has important implications, particularly given the significant physical, social, and emotional risks associated with women’s alcohol misuse (Bold, Epstein, & McCrady, 2017).
1.1. Hormonal fluctuations and mood across the menstrual cycle
Women’s drinking patterns may be impacted by hormonal changes related to their menstrual cycle. Ovarian hormones – including progesterone – circulate at relatively low concentrations during the early follicular phase, which is the first phase of the menstrual cycle occurring after menstruation. Progesterone rises during the luteal phase, after ovulation. If no pregnancy occurs, progesterone levels then fall precipitously in the late luteal phase (also referred to as the premenstrual phase), and progesterone levels remain at low levels throughout menstruation (Franz, 1988).
Literature on fluctuations in ovarian hormones and their association with alcohol consumption has focused on three areas. The first area is the examination of whether fluctuating ovarian hormones directly impact women’s drinking (i.e., do women drink more at certain points in their cycle?) (Carroll et al., 2015). The second group of studies is the exploration of how ovarian hormones affect alcohol metabolism (i.e., do hormones impact the physiological and neurological effects of alcohol in women?) (Terner & de Wit, 2006). A third area of focus is on the corollary changes in mood that occur among many women at certain phases of the menstrual cycle, and the extent to which these mood changes impact the propensity to drink. In other words, ovarian hormones affect intrapersonal processes (i.e., mood) that may increase the probability that a behavior (drinking) will be emitted (Nelson, 2010).
There is substantial evidence for the physiological effects of progesterone and its metabolites on mood. For example, the progesterone metabolites, allopregnanolone and pregnanolone, have behavioral and biochemical characteristics similar to ethanol, barbiturates, and benzodiazepines (Söderpalm, Lindsey, Purdy, Hauger, & De Wit, 2004). Allopregnanolone acts on the brain via the GABA system (Bäckström et al., 2003). Furthermore, the post-ovulatory or luteal phase has been associated with increased negative affect (Lustyk, Olson, Gerrish, Holder, & Widman, 2010) and heightened reactivity to stress (Carroll et al., 2015). Changes in negative mood could occur for several reasons, including the drop in progesterone levels (Hantsoo & Epperson, 2015), subjective expectations about negative mood during the late luteal phase (Robison & Clore, 2002), individual differences in sensitivity to hormonal changes (Hammarbäck, Damber, & Bäckström, 1989; .Hantsoo & Epperson, 2015), and/or physical discomfort that occurs for some women during the late luteal period (Tassorelli et al., 2002). Regardless of the underlying cause(s), a large percentage of women tend to experience mood changes during the late luteal phase, and among certain women, negative mood changes may increase the risk of drinking alcohol to cope (Carroll et al., 2015). Currently, there is little research to help us understand which women might be at risk of drinking to cope during certain phases of the menstrual cycle.
1.2. Progesterone, mood, and alcohol use among women
Research on individuals who drink have consistently highlighted the role of negative emotions and stress on women’s alcohol use (Karpyak et al., 2016; Mushquash et al., 2013; Nolen-Hoeksema & Hilt, 2006; Olenick & Chalmers, 1991). The “drinking-to-cope” association in women is notable, particularly given that individuals who drink to cope with negative emotions or stress are more prone to experiencing negative consequences from their alcohol use and may eventually even develop an alcohol use disorder (AUD; Campbell & Demb, 2008; Merrill & Read, 2010; Pedrelli et al., 2016). According to negative reinforcement models of alcohol use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004), and self-medication hypotheses of drinking (Khantzian, 1997), individuals drink to cope with, or get rid of, negative emotional states. As alluded to above, research suggests that the endocrine system has important implications for mood regulation in about 40% of women (Mello, Mendelson, & Lex, 1990). Decreases in progesterone may increase women’s negative affect and decrease their well-being, which may affect their proclivity to drink to cope with subsequent premenstrual symptoms. For example, in one study, 69% of treatment-seeking women under the age of fifty reported that the late luteal phase of the cycle acted as a trigger for drinking. Their retrospective reports were corroborated during treatment when women tracked their alcohol consumption and menses via daily logs (Epstein et al., 2006). Although in that study the women were not asked for the reasons why the late luteal period was a trigger for drinking, it is possible (and perhaps likely) that increased stress, negative emotions, and/or physical discomfort related to their menstrual phase played a role. Another study (Mello et al., 1990) with social drinkers found that more severe premenstrual symptoms (i.e., hostility, anxiety, impulsivity, loss of pleasure) were associated with increased alcohol consumption in eight of fourteen women. Two studies examining changes in motivations for drinking across the menstrual cycle have shown that motives for drinking to reduce tension and negative affect increase during the late luteal and menstrual phases and are related to increased drinking (Joyce et al., 2018; Sutker, Libet, Allain, & Randall, 1983). Since direct relations between hormone levels and outcomes were not tested in these studies, it is unclear whether drinking is best predicted by menstrual cycle phase itself or whether it is related to fluctuations in ovarian hormone levels, particularly progesterone. Another recent study with young social drinkers found that decreased progesterone during the late luteal phase predicted increased binge drinking when coupled with increases in estradiol (Martel, Eisenlohr-Moul, & Roberts, 2017). Although this study also examined and did not find a direct effect of mood on drinking, potential interactive effects of mood with progesterone were not examined.
This research has implications for understanding women’s alcohol misuse. As described above, some women experience the late luteal phase as a trigger for drinking (Epstein et al., 2006; Martel et al., 2017; Mello et al., 1990) and coping motives for alcohol use increase in the premenstrual phase and peak in the menstrual phase (Joyce et al., 2018). As drinking to cope with negative emotions or stress constitutes a significant risk factor for developing problems related to alcohol use (Campbell & Demb, 2008; Merrill & Read, 2010; Pedrelli, Borsari, Lipson, Heinze, & Eisenberg, 2016), women who experience mood fluctuations across the menstrual cycle may be at increased risk for alcohol misuse. However, results on drinking during the late luteal/menstrual phase of the cycle remain mixed (Carroll, Lustyk, & Larimer, 2015). Given that not all women’s moods are affected by neuroendocrine processes, and that mood changes do not always correspond with cycle phases (Mello, Mendelson, & Lex, 1990), the association between menstrual cycle phase and alcohol use in women is likely moderated by individual differences. One such factor may be whether or not a person experiences worsened mood during particular menstrual cycle phases. Furthermore, changes in levels of progesterone may act in consort with mood to predict women’s alcohol use. This negative mood-drinking association may be linked to menstrual cycle phase (e.g., due to a person’s expectations about premenstrual symptoms, for example) or may be linked to changes in hormones (e.g., due to a precipitous drop in progesterone and subsequent increases in negative mood). To contribute to the understanding of the psychological and hormonal precursors of women’s alcohol misuse, the current study sought to explore whether menstrual cycle phase, changes in levels of progesterone, and concomitant changes in mood – independently and in interaction with each other - potentiate day-to-day alcohol use.
1.3. Current study
This study is a secondary analysis of data that was originally collected to validate hormone levels in salivary samples (Cao et al., 2019). Participants provided daily diary data, which allowed us to examine the associations between daily mood and drinking in conjunction with changes in salivary levels of progesterone. We disaggregated within- and between-subject variability in daily mood ratings to isolate each woman’s day-to-day fluctuations in mood from women’s general propensity to experience positive or negative mood (Bolger & Laurenceau, 2013). Using this approach, we explored how mood interacted with changes in progesterone levels to predict if participants consumed alcohol on a particular day. We hypothesized that between-subject effects would show that women with overall more persistent negative mood would be more likely to drink when also experiencing decreases in progesterone levels. In other words, we anticipated that women who had more negative mood days overall (compared to other study participants, who reported more positive mood days) are at increased risk of drinking when progesterone levels were decreasing. However, as mentioned, there are variations in the extent to which progesterone levels and menstrual cycle phase are associated with mood changes, and mood changes at certain phases within the cycle may be due to different causes. It is possible that any of these three factors (progesterone, mood, and/or menstrual cycle phase) may be associated with alcohol use, independently or in interaction with each other, across the menstrual cycle. As we did not manipulate these factors to be able determine causality, we instead examined each of the main and interactive of these three factors - menstrual cycle phase, changes in progesterone level, and daily mood. Additionally, as every woman has her own barometer of negative and positive mood days and differences in the extent to which she experiences mood changes across the menstrual cycle, we included within-subject effects in the models. By using a daily measure of mood, we were able to examine the potential effect of these slight day-to-day changes in mood. We hypothesized that our within-subject effects would show that any given woman would be more likely to drink when she has a negative mood day (compared to her own average mood) if her progesterone levels are also dropping or decreasing; however, we did examine all main and interactive effects to help differentiate these effects.
2. Materials and methods
2.1. Participants
Forty-one female college students recruited from psychology classes at a public university participated in the study for research credit. The 41 biologically female participants were scheduled to attend two sessions in the lab; 36 (87.8%) returned for their second visit and 35 (85.4%) had completed their daily logs and were included in the analyses. The participants’ average age was 19.85 years (SD=2.63, range 18-30). Of all recruited participants, the majority identified as their race as White (n=21, 51.2%), followed by Black or African American (n=12, 29.3%), more than one race (n=3, 7.3%); Asian (n=3, 7.3%), or Pacific Islander (n=1, 2.4%); one woman declined to respond (n=1, 2.4%). Nine women identified their ethnicity as Hispanic/Latina (22.0%). One-way ANOVAs and chi-square analyses revealed no differences in age (p=.94), use of birth control (p=.48), race (p=.66), ethnicity (p=.34), or drinking variables (all p’s>.08) between women who returned and completed their logs as compared to those women who either did not return or failed to return their logs to the researcher.
2.2. Procedure
When signing up for the study, women were required to schedule two visits exactly two weeks apart. Prior to both sessions, the researcher sent an email the day prior to their participation to remind participants not to eat, drink, or brush their teeth 30 minutes prior to their scheduled appointments. In this email, participants were instructed to reschedule their participation that if they had undergone dental procedures in the previous three days. All laboratory sessions were scheduled between 10 am and 12 pm to control for diurnal variations in steroid hormones.
Upon arrival at the laboratory for Session 1, the women provided informed consent and were instructed on completing self-report questionnaires and the daily logs they would complete during the two-week period between Sessions 1 and 2. They were instructed to record the date of each log as suggested by Green and colleagues (2006). The research assistants emphasized the importance of maintaining the integrity of the daily logs while providing the instructions (e.g., completing that day, the importance of the logs to the study). Next, they were asked to swish water (approximately 4 oz) in their mouth to clear their saliva of contaminants. Ten minutes later, they provided a saliva sample (2 mL) via passive drool. All samples were immediately placed in a cooler, transferred to a −80°C freezer to maintain stability and analyzed within three months of collection. Work with progesterone and other steroid hormone analytes suggest that saliva samples stored at −80 remain stable for at least 84 days (Toone et al., 2013) and even up to four years (Salimetrics, 2019). After providing the samples, participants completed the web-based self-report questionnaires. At the end of Session 1, they were given the daily logs to complete nightly over the next two weeks. The second visit followed the same procedures, except that participants now returned the daily logs before completing a set of questionnaires and providing a second saliva sample. Then they received course credit and were debriefed.
2.3. Daily Logs
On the hard-copy, paper daily logs, women recorded: (a) day of the week, (b) “In general, how was your mood today?” (response options ranged from 1 “extremely bad/negative” to 4 “Neutral” to 7 “Excellent”) and (c) “Did you have your menstrual period today?” (Yes/No). The daily logs, and questions regarding mood, were derived from previous research among women seeking treatment for alcohol use (e.g., Holzhauer et al., 2017). Participants also recorded their answers to the question, “If someone offered you a drink, or asked you to go out drinking today, how confident are you that you would have drunk alcohol?” with the following 5 response options: (1) “Definitely would not have drunk alcohol, and did not drink today” (2) “Might have drunk alcohol, but did not drink today” (3) “Probably would have drunk alcohol, but did not drink today” (4) “Definitely would have drunk alcohol, but did not drink today” or (5) “I did drink alcohol today.” Descriptive analyses showed that participants rated 85.3% of all days as non-drinking days (options 1-4) and 14.6% as drinking days (option 5). Of the non-drinking days, the women selected that they would not have used alcohol, even if given the opportunity to drink, on 154 days (44.4%; option 1), whereas the remaining portion (n=141, 40.3%) indicated that they might have drunk if given the opportunity (option 2-4). This approach to assessing likelihood of alcohol use has been used in previous research (Jackson et al., 2014), and willingness to drink has been associated with actual drinking behavior in a young adult sample (Lewis, et al, 2016). Given the relatively lower response rates to options 2-4 across participants, we integrated the 5-item scale into three items to attain a more meaningful and interpretable outcome variable: (0) no drinking/would not have drunk (option 1; 44.0% of days recorded in the daily logs), (1) no drinking/but might have drunk if given the opportunity (options 2-4; 40.3% of days), and (2) did drink today (option 5; 14.6% of days). Our statistical model (see below for details) was run using both the 5-item and 3-item outcomes, and both the Akaike and the Bayesian Information Criterion fit statistics showed that the 3-item version was a better fit to the data in the full model (AIC=990.67→563.69, BIC=1021.14→592.94). Thus, the 3-item version was used for the analyses and reported in the results section.
2.4. Estimating Phase of Menstrual Cycle
During their first laboratory visit, the women provided the date of the first day of their most recent menstrual period and responded yes or no to a question assessment the regularity of their menstrual cycles (“Are your menstrual cycles regular?”). The date of their most recent menses was then used to derive the menstrual cycle day for each of the daily log entries, along with the women’s daily entries whether they menstruated or not during the study, using guidelines proposed by Joyce and Stewart (2018) to code menstrual phase. If a woman began her menses during the two-week study period, the start of her late luteal phase was estimated as beginning five days prior to the first day of her period; menstrual cycle phase was then estimated for each woman, for each day of the study, using the following scheme: (1) days with reported menses, (2) follicular phase: included days without reported menses, up to Day 12 of cycle; (3) ovulatory phase: included Day 13 to 16; (4) luteal phase: included Day 17 to the start of the late luteal phase; (5) late luteal phase: defined as 5 days prior to menses. For women who were on birth control, we created a sixth group (6) to account for times when they were taking active birth control (i.e. not during their menses), during which they would be maintaining steady levels of progesterone and estrogen; only their menses (experiencing a drop in ovarian hormones) was coded as usual. We opted for the phases rather than standardized menstrual cycle day proposed by Joyce and Stewart (2018) because we were primarily interested in the shifts in menstrual cycle phase as it relates to progesterone, and progesterone does not have a linear rise over the menstrual cycle. Twelve women did not have their menses while participating in the study; for those women Day 17+ was estimated as luteal phase days (i.e., 17 days after the onset of their last menses). These estimates were compared to progesterone level assays to test correspondence between progesterone levels and estimated phase. While these estimates of menstrual cycle phase follow past research methodology, it is important to note that the focus of the current study was on changes in progesterone levels (which were measured via objective hormonal level, not via estimated calendar methods). The focus was not on menstrual cycle phase, given that we did not confirm participants’ ovulation as suggested for studies focused on menstrual cycle phases specifically (Lustyk et al., 2010), but on changes in progesterone levels.
2.5. Patterns of Alcohol Use
The CORE Alcohol and Drug Survey (CORE; Presley, Meilman, & Lyerla, 1994) measures substance use in college students and was used to assess the participants’ frequency of alcohol consumption. We collected information about alcohol intake over the past month (from 1 = 0 days to 7 = all 30 days), the average number of drinks per occasion, and the number of binge drinking episodes (four or more drinks per occasion) over the previous two weeks. Alcohol use was measured during the laboratory sessions. Thus, retrospective alcohol use reported in the CORE during the second visit overlapped with the daily diaries. The means and standard deviations can be found in Table 1.
Table 1.
Alcohol use variables at baseline and during study
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|---|
| At Session One, from CORE: | |||||||||
| 1 | Number of drinks per drinking day, past 2 weeks | 3.67 | 1.90 | ||||||
| 2 | Total number of drinks in past week | 3.87 | 3.88 | .64*** | |||||
| 3 | Number of binge drinking episodes, past 2 weeks | 1.92 | 1.02 | .68*** | .73** | ||||
| 4 | Number of drinking days during the past 30 daysa | 2.87 | 1.19 | .58** | .70** | .64** | |||
| During two-week study: | |||||||||
| 5 | Mean percentage of days rated as “did not/would not drink” across women | 45.26 | 31.98 | −.38 | −.46* | −.37 | −.58** | ||
| 6 | Mean percentage of days rated as “did not/would drink” across women | 40.59 | 30.50 | .31 | .36 | .29 | .39 | −.93** | |
| 7 | Mean percentage of days rated as “did drink” across women | 14.15 | 11.69 | .24 | .31 | .25 | .57** | −.31* | −.06 |
Note: Bivariate correlations with
p<.05,
p<.01,
p<.001
Note this was on a scale from 1 (no days) to 7 (all 30 days), with a score of 2 being “1-2 days” and score of 3 being “3-5 days”
2.6. Depression, Anxiety, Stress
Participants completed the 42-item Depression Anxiety Stress Scales, DASS (Lovibond & Lovibond, 1995) at both sessions, rating the presence of symptoms over the past week on a 4-point Likert scale (from 0 = “did not apply to me at all” to 3 = “applied to me very much, or most of the time”). Scores ≥10 for depression and scores ≥8 for anxiety have been identified as the clinical cut-off (Lovibond & Lovibond, 1995). The internal consistency coefficients for the sample were .89, .88, and .83 (Cronbach’s alphas) at Session 1 and .91, .95, and .78 at Session 2 for the stress, depression, and anxiety subscales, respectively. The DASS was used in these analyses to examine how mood rating on the daily logs was associated with clinical symptoms, and to cross-validate participants’ self-reported mood on the daily logs.
2.7. Hormone Analyses
Liquid chromatography tandem-mass spectrometry was used to determine the progesterone concentrations in the saliva samples. After extraction and derivatization, the analysis was conducted using positive-ion-multiple-reaction monitoring mode of electrospray ionization tandem mass spectrometer (AB Sciex API 2000™). Derivatized progesterone was purified using C18 reverse-phase liquid chromatography and detected by monitoring the m/z 475→257 transition as the quantifier and 475→269 transition as the qualifier; HTP-[2,3,4-13C3]progesterone by monitoring the m/z 478→260 transition. The assay demonstrated the lowest limit of detection of 0.0125 ng/mL and the lowest limit of quantitation of 0.05 ng/mL at which the signal to noise ratio was ≥ 6 with coefficient of variation (CV) ≤ 25.0%. The intra-assay imprecisions CVs (n = 10) were ≤ 10.5%. The inter-assay CVs were ≤ 19.3%. Linear responses were obtained with R2 = 0.996 (0.05 and 15.0 ng/mL).
2.8. Data Analytic Plan
All analyses were conducted with SPSS v21.0 or SAS 9.4. We used a repeated-measures General Linear Models to assess the main and interactive effect of transitioning between the luteal and ovulatory cycle (i.e., when progesterone would be increasing or high) to/from any other phase (i.e., when progesterone would be low). We used an Ordered Generalized Linear Mixed Model (OGLMM) with Laplace approximation to assess the interactive effects of daily mood ratings with estimated menstrual cycle phase and then change in progesterone. Most studies investigating the effect of menstrual cycle phase on drinking have relied on calendar methods (Carroll et al., 2015; Epstein et al., 2006) like the one we used in this study. Therefore, we first assessed the effect of menstrual cycle phase (time-varying) on daily willingness to drink (Model 1), investigated the effects of change in progesterone (Model 2), and then included both menstrual phase and progesterone in the same model (Model 3).
Daily mood was grand-mean (z-scored) and person-centered (z-scored) to disaggregate the between-person differences in mood (grand-mean) from daily fluctuations within an individual (person-averaged), as suggested by Bolger and Laurenceau (2013). A mean-centered variable (based on either the grand mean or person mean) reflecting each participant’s mood for each study day was entered to analyses, providing information about day-to-day variability. By using this daily measure of mood, we were able to examine how day-to-day variations in mood might moderate the association between menstrual cycle phase/progesterone levels and the likelihood of drinking. The study hypothesized that even small, within-person changes in mood might have this moderating effect and that between-person differences in mood would have this effect as well. For instance, whether a woman is experiencing a more negative mood on a given day, compared to her mood yesterday or last week, may interact with her progesterone levels on that day to predict her alcohol use (within-person effect). Additionally, whether a woman’s mood on any given day is especially low compared to other women (e.g., at a 4 out of 7, while all other participant’s averaged score is closer to a 6) or as high (e.g., at a 7 out of 7, while all other participant’s averaged score is closer to a 4) could interact with progesterone levels and predict her alcohol use for that day. This latter measure approximates a more “trait” assessment of her daily mood, and theoretically would correlate with measures of persistent negative mood (such as depression or anxiety). The daily measure of mood was used so that the daily co-occurrence of mood changes and likelihood of alcohol use could be examined.
Time-varying effects [i.e., subject-centered mood, diary day, approximate menstrual cycle stage by day (menstrual, follicular, ovulatory, luteal, and late luteal phases), weekend vs. weekday] were modeled at the daily level (Level 1), whereas the remaining variables were measured at the person-level (Level 2) [i.e., change in progesterone across the two weeks, grand-mean-centered mood, use of non-hormonal or hormonal birth control (yes/no), past-month alcohol use]. All continuous variables were z-scored, including mood, to minimize the effects of scale differences between the variable of interest. The changes in progesterone with the between-subject (i.e., grand-mean centered) and within-subject (i.e., person-centered) mood variables were included next, followed by the full model with both progesterone change and menstrual phase and their interaction with the within- and between-subject mood variables. Mean-centered diary day was included as a fixed effect in the model to account for the effect of time. Also, we included the following random effects to account for variations at Level 1: (1) a random slope for the diary day using an autoregressive covariance structure, and (2) a random slope for within-subject mood using an unstructured covariance structure. When the random intercept was included in both models, its variance was less than 0, indicating that the means did not vary between individuals, and as such the random intercept was removed from the model. Because we used standardized continuous predictors, we could exponentiate the log-odds to generate an odds ratio that could be interpreted as an effect size. We then interpreted the magnitude of the effect using cut-offs proposed by Chen and colleagues (2010) where odds ratios (OR) of 1.68, 3.47, and 6.71 indicate small, medium, and large effects, respectively.
Planned covariates included coded variables that indicated whether each day was a weekday versus weekend, age (mean-centered), and past-month alcohol use (mean-centered). Rather than exclude women who were on birth control, we chose to include these women for three reasons: first, use of birth control methods is common among young adult women, particularly women who had higher levels of education (Mosher & Jones, 2010). Since we were targeting college-aged women drinkers, we would be excluding a substantial portion of individuals, and perhaps biasing our sample, if we focused only on women who were not taking birth control. Second, previous research has shown mixed effects of birth control on progesterone during a menstrual cycle (Schultheiss, 2003, Liening et al., 2010), and progesterone may still fluctuate and exert effects across the menstrual cycle, even if this fluctuation is dampened. Third, there is very little research on the drinking habits of women on birth control.
3. Results
3.1. Descriptive Statistics
Ten women reported not having regular menstrual cycles and were dropped from further analyses. Four individuals reported having had their last period more than 38 days prior to their participation, but otherwise reported regular cycles. Three out of these four women who had cycles longer than 35, but less than 39 days, reported beginning menses while in the study. We therefore kept these three women for analyses that investigated progesterone alone, but not for analyses using menstrual cycle phase, and completely dropped data from the one woman for whom we could not estimate her transition between cycles during the study (as she did not report menses onset during the study). Although these women fell outside the conventional 21-35 day window for what is considered a normal cycle (Joyce & Stewart, 2018), we opted to keep these women in the progesterone analyses, as there is often significant intraindividual variability in the length of cycles – as many as 50% of women report having 5-11 days variability in the length of their cycle (Münster, Schmidt, & Helm, 1992). Excluded women did not differ from women who were included in the analyses in age (t(35)=−.019, p=.99), race (χ2=2.83, p=.59), or ethnicity (χ2=1.50, p=.22).
Forty-three diary days were completed during menses (12.8%), 82 days during the follicular cycle (24.4%), 44 during the ovulatory period (13.1%), 120 during the luteal cycle (35.7%), and 47 during the late luteal phase (14.0%). The average maximum menstrual cycle length during the study was 24.5 ± 7.30 days. However, as this was a two-week study and women participated at various points in their cycle, this does not reflect the average cycle length for these women. Of those who had a full cycle during the study (n=11), the average cycle length was 31± 6.63 days. Eleven women were on hormonal or non-hormonal birth control (54.2%). Women on birth control did not differ from those not on birth control in race, χ2=3.16, p=.53 or age, t(22)=.024, p=.98. In the 30 days prior to the Session 1, three women drank no days (12.5%), seven women drank 1-2 days (29.2%), six drank 3-5 days (25.0%), six drank on 6-9 days (25.0%), and two women drank 10-19 days (8.3%). Women on birth control were not significantly different from women not taking birth control in any baseline drinking measure (p’s>.38). Most participants completed all fourteen diary days; four participants missed one day of diaries each. This final dataset consisted of 330 observations, of which 96 days occurred on a weekend.
3.2. Correspondence of Daily Log Measures with Clinical Symptoms & Past Behavior
3.2.1. Association of Participants’ Reported Daily Mood on Logs with Clinical Symptoms on DASS.
Although this was a non-clinical sample of women, we examined how women’s mood ratings over the study period were associated with clinical symptoms reported on the DASS. Four women met the clinical cut-offs for anxiety and depression on the DASS and one woman met the clinical cut-off for only anxiety. The mean mood score on daily logs within this sample was 4.80 (SD=.60). When we refer in our between-person analysis results to “more positively valenced mood,” this could be thought of as those who rated their daily mood on average one standard deviation higher than the mean, or 5.40; “more negatively valenced mood” could be thought of as those who rated their daily mood, on average one standard deviation lower than the mean, closer to 4.20. Of the women (n=4) who had a mean mood rating of 4.8 or lower, two of these women also scored above the clinical cut-offs for both the depression and anxiety subscales. We calculated bivariate correlations between mean mood ratings and scores on the three subscales of the DASS during study participation. Mean mood scores on daily logs negatively correlated with participants’ stress (p=.002), depression (p=.004), and anxiety (p=.021) during the second week of study participation (i.e., the week prior to Session 2, which overlapped with their actual daily logs). This suggests that in this non-clinical sample of women, reported mood on daily logs over the two-week period corresponded with retrospective self-reports of depression and stress on the DASS. Note that the continuous mood daily mood measure was used as a predictor in our analyses and that the DASS was administered at the two lab sessions (not only a daily basis). Given that the study goal was to examine daily variations in mood and concurrent variations in drinking, we used the daily mood measure rather than the DASS scores or clinical cut-offs, particularly since this was a non-clinical sample of college-aged women.
3.2.2. Association of Participants’ Reported Daily Drinking Logs and Past Alcohol Use.
We used bivariate correlations to examine whether participants’ reported drinking during the study period was related to drinking behavior, including alcohol misuse, in the month prior to study participation. The percent of days on which participants did not drink during the two-week daily log period was inversely related to total number of drinks in the past week (Table 1). We also calculated percent of days (during the two-week within-study period) that were rated “would drink” and “drank today”. These variables were significantly positively related to each woman’s past month drinking frequency. Thus, participants’ daily logs of drinking were associated with their typical drinking, including frequency and intensity of alcohol use. We used this past month frequency of alcohol use as a covariate in analyses to account for the effect of frequency of past drinking behavior in the main analysis.
3.2.3. Association of Changes in Progesterone Levels with Estimated Menstrual Cycle Phase.
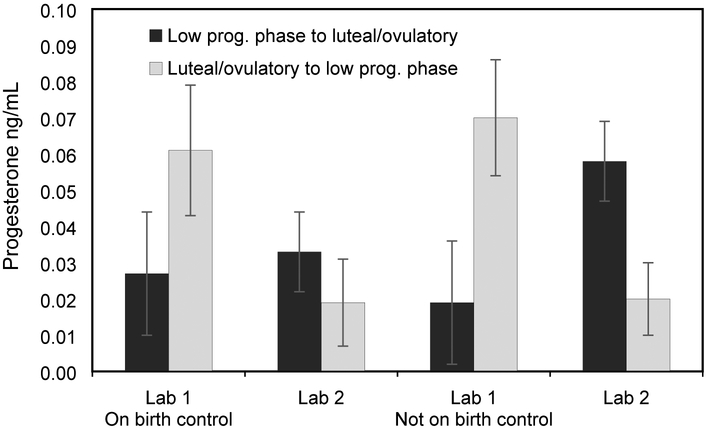
A repeated measures GLM revealed that progesterone levels changed between estimated menstrual phases as expected and did not differ based on birth control (Table 2; Figure 1). Women who transitioned from a low progesterone phase to a high progesterone phase (i.e., luteal, with one woman transitioning from the follicular to ovulatory phase) showed an increase in progesterone; whereas women who transitioned from luteal/ovulatory phase to a low progesterone phase (i.e., follicular) showed a decrease in progesterone. A second repeated measures GLM was conducted to ensure that women who had a menstrual cycle that lasted longer than 35 days displayed similar patterns of progesterone change as the other included women (Table 2). As the women with cycles longer than 35 days only transitioned from the luteal phase to the menstrual during the study, we only compared these women only to women that had a similar phase transition (out of the luteal phase to the menstrual phase). We found no difference between the three women with longer menstrual cycles as compared to those with shorter cycles.
Table 2.
Repeated-measures GLM assessing changes in progesterone.
| Model 1. Effect of birth control on changes in progesterone | ||||
|---|---|---|---|---|
| Within Effect | F | df | p | partial η2 |
| Visit | 1.171 | 1 | .292 | .055 |
| Visit X birth control | .327 | 1 | .574 | .016 |
| Visit X phase transition | 10.080 | 1 | .005 | .335 |
| Visit X phase transition X birth control | .856 | 1 | .366 | .041 |
| Between Effect | ||||
| Birth control | .544 | 1 | .469 | .026 |
| Phase transition | .789 | 1 | .385 | .038 |
| Birth control X phase transition | .042 | 1 | .840 | .002 |
|
Model 2. Effect of last menstrual cycle > 35 days in women who transitioned from luteal to another phase (n=12) on changes in progesterone | ||||
| Within Effect | F | df | p | partial η2 |
| Visit | 4.808 | 1 | .053 | .325 |
| Visit X > 35 day cycle | .000 | 1 | .984 | .000 |
| Between Effect | ||||
| > 35 day cycle | .127 | 1 | .728 | .013 |
Note: Phase transition refers women who transitioned to or from the luteal/ovulatory phases (high progesterone) to any other phase in their cycle. Model 2 was only conducted in women who transitioned from luteal/ovulatory phase to any other phase as no women who had cycles over 35 transitioned from any phase to the luteal/ovulatory phase.
Figure 1.
Progesterone levels on lab visits 1 and 2 based on estimated menstrual phase transition. Transition was coded (0) for women who experienced a transition from a lower progesterone phase to the luteal/ovulatory phases when progesterone was expected to increase or be high and (1) for women who transitioned from the luteal/ovulatory phases to a lower progesterone phase.
3.3. Main Analyses: Mixed Effects Model of Menstrual Cycle Phase, Mood, and Changes in Progesterone Predicting Alcohol Use
3.3.1. Step 1: Menstrual Cycle Phase and Mood.
Results from the OGLMM menstrual phase analysis indicated that the diary day falling on a weekend and past-month alcohol use were all significantly associated with increased odds of drinking, p’s ≤ .001. The interaction of within-subject mood with ovulatory phase relative to menses was significant (Table 3, p=.028). Being in the ovulatory phase (when progesterone is increasing) relative to the menstrual phase (when progesterone was at its lowest levels) was related to an increased likelihood of drinking when women were in a more positive mood relative to their own average mood level. A different pattern was noted when examining between-subject mood relative to menstrual cycle phase. Women who tended to have more negative mood on average were more likely to drink when they were also simultaneously experiencing their lowest level of progesterone (i.e., menses) relative to when they had higher levels of progesterone (i.e., women who were on birth control, or who were in the ovulation or luteal phase).
Table 3.
Results from Hierarchical Ordered Generalized Linear Mixed Effects Models.
| Step 1: Menstrual Phase | Step 2: Progesterone Change | Step 3: Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n=21 | n=24 | n=21 | |||||||
| Fixed effects | Est | 95% CI | p | Est | 95% CI | p | Est | 95% CI | p |
| (Intercept) Drank | −3.294 | −4.495, −2.093 | <.001 | −3.326 | −4.334, .700 | .145 | −3.715 | −5.023, −2.407 | <.001 |
| (Intercept) Would drink | −.631 | −1.735, .473 | .244 | −.358 | −1.630, 3.348 | .474 | −.909 | −2.036, .217 | .106 |
| Control variables | |||||||||
| Diary log day | −.154 | −.484, .175 | .358 | −.053 | −.305, .199 | .680 | −.102 | −.428, .224 | .540 |
| Age | −.308 | −.696, .080 | .112 | −.227 | −.524, .071 | .127 | −.329 | −.737, .078 | .106 |
| Weekend | .946 | 1.737, 3.155 | <.001 | 2.205 | 1.580, 2.829 | <.001 | 2.573 | 1.850, 3.296 | <.001 |
| Past month alcohol use | .946 | .588, 1.303 | <.001 | 1.036 | .723, 1.349 | <.001 | 1.100 | .706, 1.494 | <.001 |
| Mood | |||||||||
| BW mood | 1.216 | −.216, 2.647 | .096 | −.761 | −1.254, −.267 | .003 | .063 | −1.608, 1.733 | .941 |
| WN mood | −.805 | −2.335, .724 | .301 | .866 | .340, 1.392 | .001 | −.235 | −1.941, 1.471 | .786 |
| Menstrual Cycle | |||||||||
| Birth control vs Menstrual | .759 | −.381, 1.898 | .181 | 1.010 | −.173, 2.194 | .090 | |||
| Follicular vs Menstrual | −.729 | −2.070, .612 | .271 | −.232 | −1.611, 1.147 | .730 | |||
| Ovulatory vs Menstrual | −.114 | −1.615, 1.386 | .876 | .537 | −1.062, 2.137 | .493 | |||
| Luteal vs Menstrual | .387 | −.897, 1.670 | .538 | 1.057 | −.288, 2.402 | .117 | |||
| Late Luteal vs Menstrual | −.269 | −1.819, 1.281 | .722 | .226 | −1.401, 1.853 | .776 | |||
| Birth control vs Menstrual X BW mood | −2.073 | −4.019, −.128 | .037 | −.662 | −2.850, 1.527 | .552 | |||
| Follicular vs Menstrual X BW mood | −1.909 | −4.600, .781 | .164 | −1.108 | −4.165, 1.948 | .476 | |||
| Ovulatory vs Menstrual X BW mood | −4.179 | −7.343, −1.015 | .010 | −2.386 | −5.720, .948 | .160 | |||
| Luteal vs Menstrual X BW mood | −2.315 | −4.336, −.293 | .025 | −1.020 | −3.253, 1.214 | .369 | |||
| Late luteal vs Menstrual X BW mood | −1.067 | −2.983, .849 | .274 | −1.180 | −3.365, 1.005 | .288 | |||
| Birth control vs Menstrual X WN mood | 1.943 | −.090, 3.975 | .061 | .974 | −1.268, 3.216 | .393 | |||
| Follicular vs Menstrual X WN mood | 1.697 | −.548, 3.941 | .138 | 1.280 | −1.100, 3.661 | .291 | |||
| Ovulatory vs Menstrual X WN mood | 3.318 | .356, 6.279 | .028 | 2.599 | −.517, 5.714 | .102 | |||
| Late luteal vs Menstrual X WN mood | 1.793 | −.102, 3.688 | .064 | 1.317 | −.654, 3.289 | .189 | |||
| Birth control vs Menstrual X WN mood | 1.074 | −1.176, 3.324 | .348 | 1.198 | −1.360, 3.757 | .357 | |||
| Progesterone | |||||||||
| Progesterone Δ | −.275 | −.574, .024 | .069 | −.478 | −.902, −.055 | .029 | |||
| BW mood X Prog Δ | −1.005 | −1.481, −.529 | <.001 | −1.153 | −1.816, −.490 | .001 | |||
| WN mood X Prog Δ | .654 | .103, 1.204 | .020 | .637 | −.061, 1.334 | .074 | |||
Note: Estimates are in log-odds. All continuous variables are standardized to minimize effects of scale and aid in interpretation. CI=Confidence Intervals; BW = Between participant effects; WN = Within participant effects.
3.3.2. Step 2: Mood, and Changes in Progesterone.
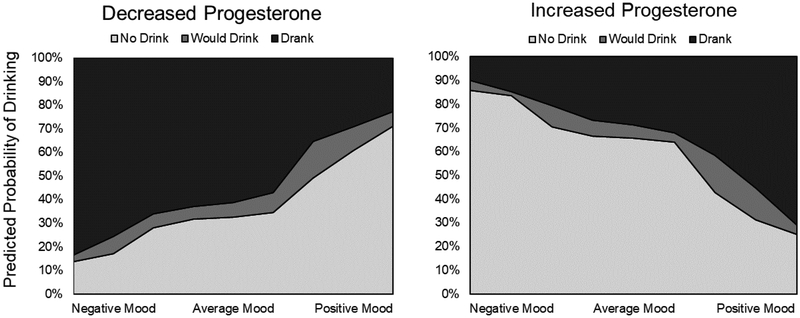
Change in progesterone and its interactions with within- and between-subject mood were run in a separate model without menstrual cycle. Although the main effect of progesterone was not significant (p=.069), progesterone tended to be protective against drinking. The interactions of change in progesterone with both mood variables were significant (p’s≤.02), albeit in different directions (Table 3). A woman was more likely to drink when decreases in progesterone occurred on the same days she was also experiencing a decrease in her mood (i.e., an increase in negative mood, relative to her own average mood) (Figure 2). In other words, when a woman was having a negative mood day (compared to her own “baseline”, or average mood), she was more likely to drink if also experiencing a decrease in progesterone levels (OR=1.92, or small effect). However, during an increase in progesterone, she was more likely to drink if she was in a positive mood (Figure 2).
Figure 2.
The interaction of within-individual changes in mood with changes in progesterone. Mood is centered at individual’s mood and each point is graphed at 10th percentile intervals. Progesterone is graphed at minimum and maximum values of progesterone
At the between-person level, women who had a more positively valenced mood during the two-week study period (compared to other women in the study) had 2.73 greater odds (a small-to-medium effect) to drink when their progesterone decreased and were less likely to drink when progesterone levels increased (Figure 3). In contrast, women who tended to have a more negative mood during the two-week period were more likely to drink when their progesterone level increased and were less likely to drink when their progesterone levels decreased (Figure 3). In other words, women with overall more positive mood were more likely to drink when their progesterone levels were decreasing and women who reported an overall more negative mood were more likely to drink when their progesterone levels were increasing. Thus, fluctuations in progesterone levels were associated with drinking, but the results differed based on variations in mood (at the between-subjects and within-subject levels). These moderating effects of mood on the relationship between progesterone levels and drinking were not at the clinical level – rather, within a non-clinical sample, individual differences in mood were found to impact these associations.
Figure 3.
The interaction of between-woman differences in mood (fixed variable, across time) and change in progesterone. Mood is centered at the grand mean and graphed at the minimum and maximum values. Each point for change in progesterone is graphed at 10th percentile intervals.
3.3.3. Step 3: Menstrual Cycle Phase, Mood, and Changes in Progesterone.
When tested in a combined model, with changes in progesterone and menstrual cycle phase both entered simultaneously (Table 3). The protective main effect of progesterone became significant in this model (OR=1.61, small effect). The interactive effect of between-subject mood progesterone with mood remained significant (p=.001) but the interactive effect of within-subject mood with progesterone and of both within- and between-subject mood with menstrual cycle was no longer significant.
4. Discussion
In the current study, we examined how progesterone, menstrual cycle, and women’s daily mood were associated with daily alcohol use. A large percentage of women report the late luteal phase as a trigger for alcohol use, which has been attributed to changes in mood and stress reactivity that can occur during this time (Lustyk et al., 2010). In our study, we found support for these findings – women were more likely to drink during their menses than in higher progesterone menstrual phases (ovulatory or luteal) – however, this finding was specific to women who experienced an overall more positive mood (compared to women with less-than-average daily mood). Additionally, we found that natural fluctuations in progesterone significantly interacted with women’s daily mood to statistically predict drinking on that particular day. That is, we found support for our hypothesis that women were more likely to drink on days they were experiencing a decrease in progesterone if also experiencing a negative mood (compared to their average mood). Although main effects suggested that women were more likely to drink on days they were in a better mood, this was not the case for women who were experiencing decreasing levels of progesterone. This supports findings from Martel and colleagues (2017) that young women who drink socially were more likely to binge drink on weekends when they were also experiencing decreasing levels of progesterone. While our study did not assess binge drinking, our results similarly showed that young adult women are more likely to drink on days when they are experiencing decreasing levels of progesterone. We extended those results by showing that women experiencing a negative mood were more likely to drink in the context of a decrease, but not an increase, in progesterone levels. These results, combined with the findings from the menstrual phase findings, tentatively suggest that increasing progesterone may act as a protective factor by decreasing the likelihood of women experiencing, or drinking to cope with, negative emotions. Alternatively, women experiencing increases in progesterone were more likely to drink on days they were in a relatively positive mood. Although this was unexpected, it is consistent with research showing that mood enhancement motives are a strong predictor of drinking among college-aged individuals (Read, Wood, Kahler, Maddock, & Palfai, 2003). However, other research in young adult women has shown that enhancement motives do not fluctuate across the menstrual cycle (Joyce et al., 2018). Therefore, the association between positive mood, increases in progesterone, and alcohol use may be due to other reasons, such as for celebratory reasons that are unrelated to specifically drinking for the sole purpose of enhancement. Regardless, much of the research on women’s alcohol use across the menstrual cycle has focused on negative mood, but these results point to the merits of investigating both positive and negative mood as moderators of the relationship between menstrual cycle and alcohol use particularly among a non-clinical sample.
Notably, the main effects indicated that a decrease in progesterone was associated with a greater likelihood of drinking overall, further supporting that the decline of progesterone may potentiate alcohol use in women. Without manipulating women’s levels of progesterone, we cannot say whether the negative mood was due to changes in progesterone, but our findings support the assumption that the co-occurrence of these factors is associated with an increased likelihood of drinking.
An additional and novel aspect of this study was that the analyses included both within- and between-subject averaged mood in the models, and that we examined mood using a daily assessment. This allowed us to isolate effects that occurred due to each woman’s proximal mood from those due to the sample’s general emotional climate. Indeed, a different pattern of results emerged from analyses examining between-subject mood as a predictor along with its interaction with progesterone. In sum, these analyses showed that women who were “happier” during the study – who rated their daily mood as better than average (and also had lower scores on the DASS, suggesting that these women not only had generally more positive mood, but also had less corresponding clinical symptoms of depression or anxiety) – were less likely to drink in the context of increases in progesterone. But these women were still more likely to drink when they experienced a decrease in progesterone. These results suggest that for women who overall experience a better mood, increasing levels of progesterone may serve as a protective factor in terms of drinking.
In contrast, women who on average had experienced a more negative mood across the study period (and had higher corresponding depression/anxiety scores on the DASS) were less likely to drink in the context of decreasing progesterone levels and more likely to drink when progesterone levels increased. Although daily negative mood and decreasing progesterone levels were associated with drinking in all women, those with a more negative mood overall were less likely to drink in conjunction with decreasing levels of progesterone. These women who rated their mood more negatively also reported more symptoms of stress, depression, and anxiety during the two-week period and prior to study participation.. These findings are consistent with the assumption that women with more negative mood overall in a non-clinical sample may be more prone to using ineffective coping behaviors such as rumination rather than drinking alcohol (Nolen-Hoeksema, 2012). However, even these women were more likely to drink when they showed increasing daily negative mood ratings in the context of decreasing progesterone levels, just not in comparison to other women who reported more positive mood overall. Again, these results were unexpected but suggest that women’s levels of internalizing symptoms may moderate the association between hormonal fluctuations and their propensity to use alcohol. Women with co-occurring internalizing symptoms may not experience the protective effects of increasing levels of progesterone to the same extent as other women. Conversely, there may be less overall variability in their mood so that they are less sensitive to any mood-related effects of progesterone. This finding may also have important implications based on recent research that shows women with post-traumatic stress disorder demonstrate a “block” in their conversion of progesterone to allopregnanolone, the metabolite largely responsible for progesterone’s anxiolytic effects (Pineles et al., 2018).
We chose to include women who were on birth control in our analyses because these women represent a significant portion of women who are in college (Buhi et al., 2010, Huber & Ersek, 2009). We found that these women continued to experience fluctuations in their progesterone, consistent with other studies (Liening et al., 2010). Women on birth control were more likely to drink during their menstrual phase compared to all other days of their menstrual cycle, particularly among women with overall more negative mood (compared to those with overall more positive mood). Thus, the results from this study, while preliminary, suggest an association between menstrual cycle phase, mood, and drinking even among women on birth control. However, this effect of menstrual cycle phase was no longer significant after entering progesterone levels, furthering the finding that changes in progesterone may be predictive of drinking among women, above and beyond menstrual cycle phase.
The current study asked the young adult participants about their thoughts on drinking and whether they would drink if provided the opportunity. The goal was to collect data on their willingness to drink because the lack of availability of alcohol may have prevented them from drinking even if they would have otherwise drunk on a particular day. Our results support this approach, as the women rated about 43% of all study days as days they might have drunk if given the option, whereas only 15% of days were actual drinking days. However, while the option “may have drunk, but didn’t” appeared to be a popular option for the participants, it did not figure largely into the analyses or results. The predictors still primarily differentiated between days on which women drank alcohol versus days on which they did not drink, i.e., they differentiated based on actual drinking behavior. Thus, we could have asked about alcohol use only and have found the same results. Reporting these more detailed findings related to thoughts about drinking may inform future research but largely was irrelevant in our current analyses.
An additional strength of this study was the use of liquid chromatography mass spectrometry (LC/MS-MS), considered the gold standard in steroid hormone measurement, to directly measure levels of progesterone. Many psychological studies have relied on immunoassay techniques to measure salivary hormone levels, despite increasing evidence from the clinical chemistry field demonstrating that steroid hormones are prone to cross-reactivity between compounds of similar molecular structure (Krasowski et al., 2014; Miller, Plessow, Rauh, Gröschl, & Kirschbaum, 2013) and the inflation of hormone levels (Welker et al., 2016). By measuring progesterone directly via LC/MS-MS, we can be assured that the observed changes in progesterone levels are due to temporal variations in the steroid hormone rather than measurement error.
It is important to note several limitations of the current project. First, we do not know the extent to which these findings extend to actual misuse of alcohol or the development of AUD. This study identified associations between changing progesterone levels, mood, and alcohol use, but did not directly examine the intensity of drinking episodes, negative consequences from alcohol use, or whether the findings extend to AUD-related symptoms and behaviors. A related limitation is that, although we used a standardized coding of menstrual cycle phases, we assumed that women experienced ovulation between days 13-16 without confirmation via an ovulation test. Also, women’s menstrual cycle and hormonal fluctuations vary both between women and across cycles. A challenge for this area of research is to understand how results may be affected by regularity of menstrual cycle, something that could be addressed with more frequent hormonal measurements and longer study design. Given that this study collected samples of salivary progesterone twice during the study, while still an improvement over much of the literature in this area, more frequent measurements in a larger study would certainly benefit this area of research (Carroll et al., 2015). Although our daily measures were based on work by previous research in our own and other labs (e.g., Holzhauer, Epstein, Hayaki, Marinchak, McCrady & Cook, 2017; Park, Armeli, & Tennen, 2004; Jackson et al., 2017), the measures were not validated and were one-question assessments. For example, our mood measure was a simple assessment of overall daily mood on a 7-point Likert scale and it does not provide as fine-grained an assessment of mood. In addition, we used a paper diary to record nightly mood and willingness to drink. It would be important for future studies to use electronic diaries, which allow for each entry to be timestamped to ensure compliance with the diary. Lastly, we neither measured progesterone levels daily nor did we measure other potentially relevant sex hormones (specifically, estradiol). We showed that progesterone levels combined with mood were associated with drinking, and accounting for estradiol (or the ratio of progesterone and estradiol; e.g., see Martel, Eisenlohr-Moul, & Roberts, 2017) may further illuminate this effect. It is important to note that we did not assess if the birth control was hormonal or non-hormonal. An area for future study would be to determine if the association between progesterone fluctuations and drinking varies based on whether women are taking hormonal birth control, a question largely unknown.
4.1. Conclusions
Past research on mood and hormone fluctuations as contributors to alcohol use among women has demonstrated a plausible basis for continued research. Although the literature has shown mixed findings, researchers are now able to use advanced statistical and methodological approaches to more fully capture patterns that occur in the natural environment, which allows them to tease apart within-subject from between-subject effects. It is well known that menstrual cycle and hormone functioning can vary substantially from woman to woman, a complexity that is further complicated by the prevalent use of hormonal contraception. We found that directly measuring levels of progesterone, as opposed to using the proxy measurement of menstrual cycle phase, was more closely associated with drinking behavior. However, more research is needed, particularly given the rising rates of AUD in women and the large number who identify their menstrual cycle and associated mood changes as relevant factors in their alcohol use. Recent research suggests an association between estrogen levels and impulsive actions in women (Weafer & de Wit, 2014), and another study found that motives for drinking also vary across the menstrual cycle (Joyce et al., 2018). Thus, potential moderators of the associations between progesterone and drinking – which are areas of future research – include types of hormonal contraception use, estrogen levels, trait-based measures of impulsivity, as well as cognitive factors such as coping motives. Indeed, fluctuations in ovarian hormones may be related to women’s alcohol use in many ways, including via their propensity to increase negative mood, stress reactivity, and impulsivity. Mixed findings on the relationship between hormones and drinking among women could reflect the presence of such moderators. It is a promising area of research in need of further work. If women are at increased risk of alcohol misuse during certain phases of their menstrual cycle, these biological processes need to be considered, particularly when treating alcohol misuse. Thus, prevention and intervention efforts may need to consider women’s sensitivity to hormonal fluctuations and their emotional and cognitive correlates, help women develop coping skills to deal more effectively with these hormonal changes, and consider the use of medications to regulate hormonal imbalances.
Highlights.
Women were more likely to drink when in their late luteal or menstrual phase than in their luteal phase when coupled with an overall more positive mood.
Change in progesterone interacted with women’s daily mood to predict the likelihood of drinking.
Women were more likely to drink on days they experienced a negative mood and a decrease in progesterone.
Increasing progesterone may decrease the likelihood of women experiencing, or drinking to cope with, negative emotions.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, … Allen SS (2016). Determining menstrual phase in human biobehavioral research: A review with recommendations. Experimental and Clinical Psychopharmacology, 24(1), 1–11. 10.1037/pha0000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström T, Andreen L, Birzniece V, Björn I, Johansson IM, Nordenstam-Haghjo M, … Zhu D (2003). The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs, 17(5), 325–342. 10.2165/00023210-200317050-00003 [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction Motivation Reformulated: An Affective Processing Model of Negative Reinforcement. Psychological Review, 111(1), 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Bold KW, Epstein EE, & McCrady BS (2017). Baseline health status and quality of life after alcohol treatment for women with alcohol dependence. Addictive Behaviors, 64, 35–41. 10.1016/j.addbeh.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, & Laurenceau J (2013). Intensive longitudinal methods: An introduction to diary and experience sampling research. New York: Guilford Press; US. [Google Scholar]
- Buhi ER, Marhefka SL, & Hoban MT (2010). The state of the union: Sexual health disparities in a national sample of US college students. Journal of American College Health, 58(4), 337–346. [DOI] [PubMed] [Google Scholar]
- Campbell CM, & Demb A (2008). College high risk drinkers: Who matures out? And who persists as adults? Journal of Alcohol and Drug Education, 52(1), 19–46. [Google Scholar]
- Cao ZT, Wemm SE, Han L, Spink DC, & Wulfert E (2019). Noninvasive determination of human cortisol and dehydroepiandrosterone sulfate using liquid chromatography-tandem mass spectrometry. Analytical and bioanalytical chemistry, 411(6), 1203–1210. [DOI] [PubMed] [Google Scholar]
- Carroll HA, Lustyk MKB, & Larimer ME (2015). The relationship between alcohol consumption and menstrual cycle: a review of the literature. Archives of Women’s Mental Health, 18(6), 773–781. 10.1007/s00737-015-0568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, & Chen S (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics: Simulation and Computation, 39(4), 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- Epstein EE, Rhines KC, Cook S, Zdep-Mattocks B, Jensen NK, & McCrady BS (2006). Changes in alcohol craving and consumption by phase of menstrual cycle in alcohol dependent women. Journal of Substance Use, 11(5), 323–332. 10.1080/14659890500419717 [DOI] [Google Scholar]
- Fox HC, & Sinha R (2009, April). Sex differences in drug-related stress-system changes: Implications for treatment in substance-abusing women. Harvard Review of Psychiatry. 10.1080/10673220902899680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz WB 3rd (1988). Basic review: endocrinology of the normal menstrual cycle. Primary care, 15(3), 607. [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, … Hasin DS (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Rafaeli E, Bolger N, Shrout PE, & Reis HT (2006). Paper or plastic? Data equivalence in paper and electronic diaries. Psychological methods, 11(1), 87. [DOI] [PubMed] [Google Scholar]
- Hammarbäck S, Damber JE, & Bäckström T (1989). Relationship between symptom severity and hormone changes in women with premenstrual syndrome. The Journal of Clinical Endocrinology & Metabolism, 68(1), 125–130. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, & Epperson CN (2015). Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Current Psychiatry Reports, 17(11), 87 10.1007/s11920-015-0628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LRB, & Ersek JL (2009). Contraceptive use among sexually active university students. Journal of Women's Health, 18(7), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Roberts ME, Colby SM, Barnett NP, Abar CC, Merrill JE (2014). Wilingness to drink as a function of peer offers and peer norms in early adolescence. Journal of Studies on Alcohol and Drugs, 75, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce KM, Hudson A, O’Connor R, Thompson K, Hodgin M, Perrot T, & Stewart SH (2018). Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depression and Anxiety, 35(4), 313–320. 10.1002/da.22699 [DOI] [PubMed] [Google Scholar]
- Joyce KM, & Stewart SH (2019). Standardization of menstrual cycle data for the analysis of intensive longitudinal data In Lutsenko OI, Menstrual Cycle (pp.1–17). London, UK: Intech Open Science; DOI: 10.5772/intechopen.81504 [DOI] [Google Scholar]
- Karpyak VM, Biernacka JM, Geske JR, Abulseoud OA, Brunner MD, Chauhan M, … Mrazek DA (2016). Gender-specific effects of comorbid depression and anxiety on the propensity to drink in negative emotional states. Addiction, 111(8), 1366–1375. 10.1111/add.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1997). The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard review of psychiatry, 4(5), 231–244. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, & Ekins S (2014). Cross-reactivity of steroid hormone immunoassays: Clinical significance and two-dimensional molecular similarity prediction. BMC Clinical Pathology, 14(1), 33 10.1186/1472-6890-14-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, King K, Litt D, Swanson A, Lee C (2016). Examining daily variability in willingness to drink in relation to underage young adult alcohol use. Addictive Behaviors, 61, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, & Schultheiss OC (2010). Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & behavior, 99(1), 8–16. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, & Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-U [DOI] [PubMed] [Google Scholar]
- Lustyk MKB, Olson KC, Gerrish WG, Holder A, & Widman L (2010). Psychophysiological and neuroendocrine responses to laboratory stressors in women: Implications of menstrual cycle phase and stressor type. Biological Psychology, 83(2), 84–92. 10.1016/j.biopsycho.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Martel MM, Eisenlohr-Moul T, & Roberts B (2017). Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. Journal of Abnormal Psychology, 126(8), 1104–1113. 10.1037/abn0000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, & Lex BW (1990). Alcohol use and premenstrual symptoms in social drinkers. Psychopharmacology, 101(4), 448–455. 10.1007/BF02244221 [DOI] [PubMed] [Google Scholar]
- Merrill JE, & Read JP (2010). Motivational Pathways to Unique Types of Alcohol Consequences. Psychology of Addictive Behaviors, 24(4), 705–711. 10.1037/a0020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Plessow F, Rauh M, Gröschl M, & Kirschbaum C (2013). Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology, 38(1), 50–57. 10.1016/j.psyneuen.2012.04.019 [DOI] [PubMed] [Google Scholar]
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 23(29). 2010. [PubMed] [Google Scholar]
- Münster K, Schmidt L, & Helm P (1992). Length and variation in the menstrual cycle—a cross-sectional study from a Danish county. BJOG: An International Journal of Obstetrics & Gynaecology, 99(5), 422–429. [DOI] [PubMed] [Google Scholar]
- Mushquash AR, Stewart SH, Sherry SB, Sherry DL, Mushquash CJ, & MacKinnon AL (2013). Depressive symptoms are a vulnerability factor for heavy episodic drinking: A short-term, four-wave longitudinal study of undergraduate women. Addictive Behaviors, 38(5), 2180–2186. 10.1016/j.addbeh.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Nelson RJ (2010). Hormones and Behavior: Basic Concepts In Encyclopedia of Animal Behavior (pp. 97–105). Elsevier; 10.1016/B978-0-08-045337-8.00236-9 [DOI] [Google Scholar]
- Nolen-Hoeksema S (2004). Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review. 10.1016/j.cpr.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2012). Emotion Regulation and Psychopathology: The Role of Gender. Annual Review of Clinical Psychology, 8(1), 161–187. 10.1146/annurev-clinpsy-032511-143109 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Hilt L (2006). Possible contributors to the gender differences in alcohol use and problems. Journal of General Psychology, 133(4), 357–374. 10.3200/GENP.133.4.357-374 [DOI] [PubMed] [Google Scholar]
- Olenick NL, & Chalmers DK (1991). Gender-specific drinking styles in alcoholics and nonalcoholics. Journal of Studies on Alcohol, 52(4), 325–330. 10.15288/jsa.1991.52.325 [DOI] [PubMed] [Google Scholar]
- Park CL, Armeli S, & Tennen H (2004). The daily stress and coping process and alcohol use among college students. Journal of Studies on Alcohol, 65, 126–135. [DOI] [PubMed] [Google Scholar]
- Pedrelli P, Borsari B, Lipson SK, Heinze JE, & Eisenberg D (2016). Gender Differences in the Relationships Among Major Depressive Disorder, Heavy Alcohol Use, and Mental Health Treatment Engagement Among College Students. Journal of Studies on Alcohol and Drugs, 77(4), 620–628. 10.15288/jsad.2016.77.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, … Rasmusson AM (2018). PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology, 93(April), 133–141. 10.1016/j.psyneuen.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Presley CA, Meilman PW, & Lyerla R (1994). Development of the Core Alcohol and Drug Survey: Initial Findings and Future Directions. Journal of American College Health, 42(6), 248–255. 10.1080/07448481.1994.9936356 [DOI] [PubMed] [Google Scholar]
- Rahav G, Wilsnack R, Bloomfield K, Gmel G, & Kuntsche S (2006). The influence of societal level factors on men’s and women’s alcohol comsumption and alcohol problems. Alcohol and Alcoholism, 41(SUPPL. 1), i47–i55. 10.1093/alcalc/agl075 [DOI] [PubMed] [Google Scholar]
- Read JP, Wood MD, Kahler CW, Maddock JE, & Palfai TP (2003). Examining the role of drinking motives in college student alcohol use and problems. Psychology of Addictive Behaviors, 17(1), 13–23. 10.1037/0893-164X.17.1.13 [DOI] [PubMed] [Google Scholar]
- Robinson MD, & Clore GL (2002). Belief and feeling: evidence for an accessibility model of emotional self-report. Psychological bulletin, 128(6), 934. [DOI] [PubMed] [Google Scholar]
- Salimetrics, LLCC. 2019. Saliva collection handbook. State College, PA. [Google Scholar]
- Schultheiss OC, Dargel A, & Rohde W (2003). Implicit motives and gonadal steroid hormones: Effects of menstrual cycle phase, oral contraceptive use, and relationship status. Hormones and Behavior, 43(2), 293–301. [DOI] [PubMed] [Google Scholar]
- Söderpalm AH ., Lindsey S, Purdy RH, Hauger R, & De Wit H (2004). Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology, 29(3), 339–354. 10.1016/S0306-4530(03)00033-7 [DOI] [PubMed] [Google Scholar]
- Sutker PB, Libet JM, Allain AN, & Randall CL (1983). Alcohol use, negative mood states, and menstrual cycle phases. Alcoholism: Clinical and Experimental Research, 7(3), 327–331. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Sandrini G, Cecchini AP, Nappi RE, Sances G, & Martignoni E (2002). Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosomatic Medicine, 64(4), 621–626. [DOI] [PubMed] [Google Scholar]
- Terner JM, & de Wit H (2006). Menstrual cycle phase and responses to drugs of abuse in humans. Drug and Alcohol Dependence, 84(1), 1–13. 10.1016/j.drugalcdep.2005.12.007 [DOI] [PubMed] [Google Scholar]
- Toone RJ, Peacock OJ, Smith AA, Thompson D, Drawer S, Cook C, & Stokes KA (2013). Measurement of steroid hormones in saliva: Effects of sample storage condition. Scandinavian journal of clinical and laboratory investigation, 73(8), 615–621. [DOI] [PubMed] [Google Scholar]
- Tuchman E (2010). Women and addiction: The importance of gender issues in substance abuse research. Journal of Addictive Diseases, 29(2), 127–138. 10.1080/10550881003684582 [DOI] [PubMed] [Google Scholar]
- Weafer J, & de Wit H (2014). Sex differences in impulsive action and impulsive choice. Addictive Behaviors, 39(11), 1573–1579. 10.1016/j.addbeh.2013.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker KM, Lassetter B, Brandes CM, Prasad S, Koop DR, & Mehta PH (2016). A comparison of salivary testosterone measurement using immunoassays and tandem mass spectrometry. Psychoneuroendocrinology, 71, 180–188. 10.1016/j.psyneuen.2016.05.022 [DOI] [PubMed] [Google Scholar]