Table 2.
Scope of Directing-Group Effects in Ti-Catalyzed [2+2+1] Pyrrole Synthesis
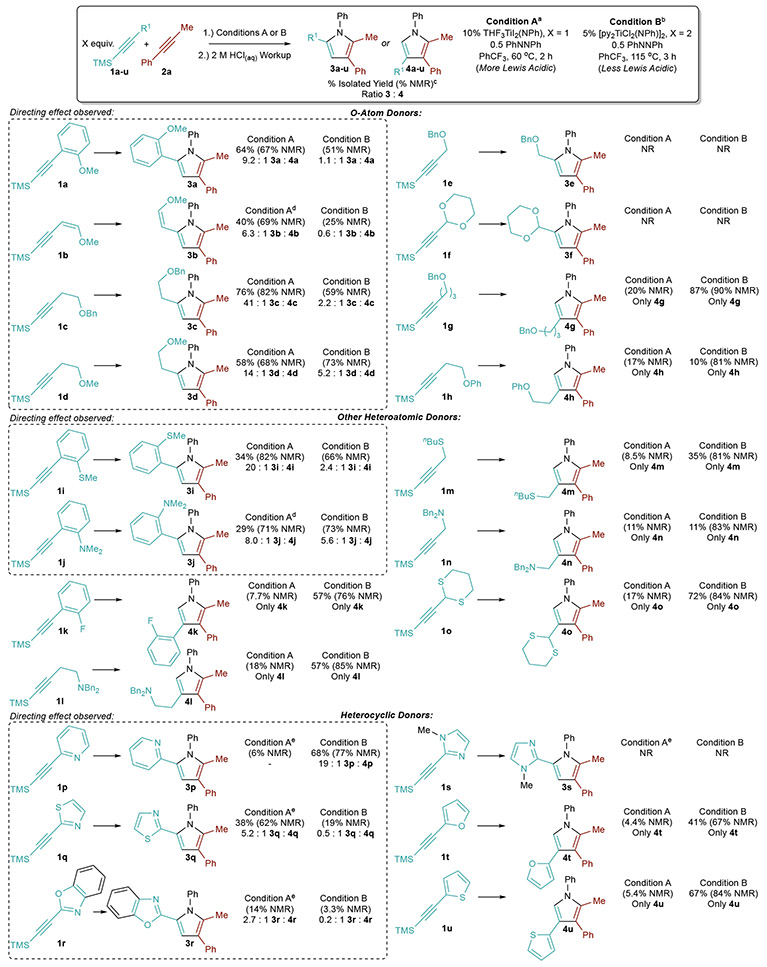
|
Condition A: A mixture of 0.5 mmol 1 (1.1 equiv), 0.5 mmol 2 (1.1 equiv), 0.225 mmol PhNNPh (0.5 equiv) and 0.05 mmol THF3I2Ti(NPh) (0.1 equiv) in 2.5 mL CF3Ph was heated for 2 h. Reactions were quenched with 2 M HCl in MeOH;
Condition B: A mixture of 1.0 mmol 1 (2.2 equiv), 0.5 mmol 2 (1.1 equiv), 0.225 mmol PhNNPh (0.5 equiv) and 0.025 mmol [py2Cl2TiNPh]2 (0.05 equiv) in 2.5 mL CF3Ph was heated for 3 h. Reactions were quenched with 2 M HCl in MeOH;
Isolated and/or NMR yield of major regioisomer drawn and are reported with respect to 2a.
Reaction run at 80 °C;
Reaction run at 115 °C.
