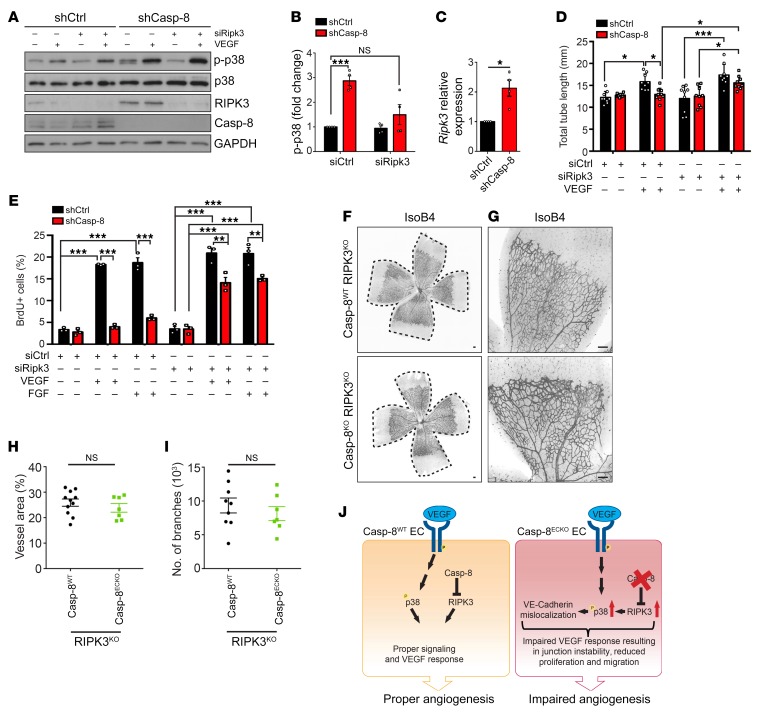
Figure 7. RIPK3 acts downstream of Casp-8 to regulate angiogenesis.
(A) Western blot showing that knocking down RIPK3 in Casp-8KD ECs rescues the basal hyperphosphorylation of p38. Notice that shCasp-8 HUVECs have increased RIPK3 protein levels. (B) Quantification of Western blots under basal conditions as in A. n = 4. (C) Quantitative PCR (qPCR) of shCtrl or shCasp-8–infected HUVECs showing increased mRNA expression of Ripk3 in the absence of Casp-8. n = 4. (D) Quantification of total tube length of shCtrl or shCasp-8–infected HUVECs that were cotransfected with control siRNA or Ripk3 siRNA and treated with or without VEGF (50 ng/mL) for 4 hours. Ten fields per condition were quantified. n = 3. (E) BrdU+ cells were quantified in control and Casp-8KD ECs transfected with Ripk3 siRNA and with or without VEGF (50 ng/mL) or FGF (50 ng/mL) stimulation for 24 hours. Around 50 cells per condition were quantified. n = 3. (F and G) Representative images of the retinal vasculature stained with IsoB4 in Casp-8WT/RIPK3KO and Casp-8ECKO/RIPK3KO mice. (H and I) Quantitative analysis showing no differences in vessel area (H; n = 11 WT, n = 7 ECKO) and number of branches (I; n = 9 WT, n = 7 ECKO). Data from 4 independent litters. (J) Working model summarizing the role of Casp-8 as a modulator of angiogenesis. Casp-8 inhibits RIPK3 to allow the proper response of ECs to VEGF stimulation (Casp-8WT). If Casp-8 is absent (Casp-8ECKO), increased RIPK3 levels induce p38 hyperphosphorylation, which in turn leads to an impaired response to VEGF stimulation and reduced angiogenesis. For B–D, E, H, and I, data are shown as mean ± SEM. *P < 0.05; ***P <0.001, 1-sample t test (B and C); *P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA with Tukey’s multiple comparisons test (D and E); 2-tailed unpaired Student’s t test (H and I).