Figure 2.
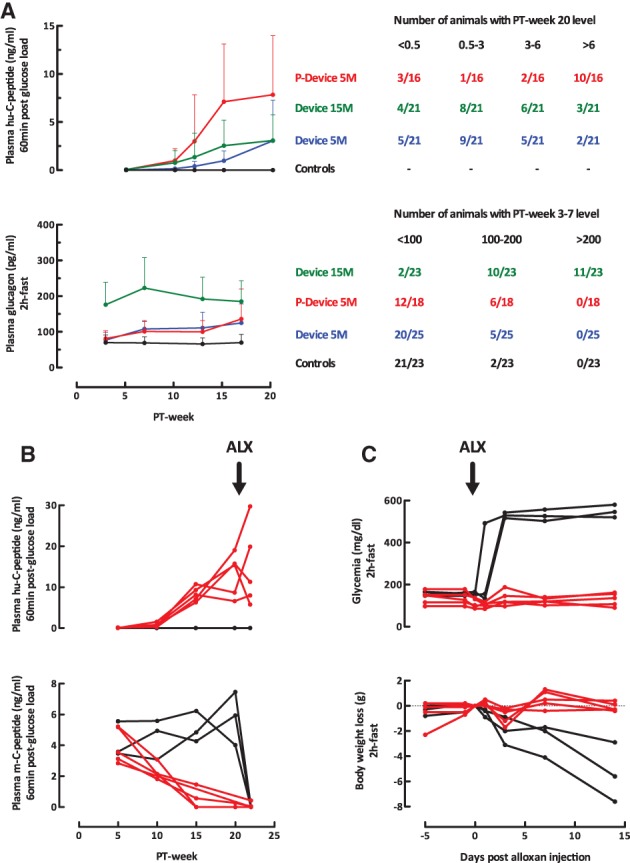
In vivo markers of implant function in recipients of device‐encapsulated human induced pluripotent stem cell‐derived pancreatic endoderm. (A): Plasma human (hu‐)C‐peptide levels (60 minutes postglucose load, 3 g/kg body weight, intraperitoneal) and plasma glucagon levels (basal, after 2 hours fast). Left part shows averages ± SD over the 20‐week follow‐up period of subgroups with 5 or 15 million cells at start with or without preformed pouch (P). Plasma hu‐C‐peptide <0.1 ng/ml (assay limit of detection) was considered as zero. Right part tabulates number of animals per subgroup according to the change in hormone levels. (B, C): Recipients of P‐Device with plasma hu‐C‐peptide levels >6 ng/ml (n = 5; red curves) and age‐matched controls (n = 3; black curves) were injected with alloxan (50 mg/kg BW) at post‐transplant week 20 and followed for plasma hu‐C‐peptide and mouse (m‐)C‐peptide levels (60 minutes postglucose load), basal glycemia, (2 hours fast) and body weight.
