Abstract
We evaluated the results of an acceptability survey administered online to users of IWTK, which offers internet-requested kits for sexually transmitted infection (STI) screening. User satisfaction was high for IWTK, with many users in our survey being repeat patients of this program. Both male and female respondents preferred genital self-collected swabs over urine collected specimens. Strong interest was expressed in home testing options for other STIs.
Keywords: home testing, point of care testing
Short summary:
A survey of I Want the Kit users shows high satisfaction with the program, and interest in expanding to point of care testing and testing for other STIs.
The I Want the Kit program (IWTK) www.iwantthekit.org offers internet-requested kits for in-home sample collection for screening of three common STIs: Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. A previous user survey for IWTK was compiled in 2006.1 Since this original user survey, the program has expanded to include men, rectal swab testing, and the website has been updated to allow individuals to obtain their own results. Our updated survey was planned to continue to monitor user acceptability of the program, and to gauge interest for future directions for testing for other STIs.
METHODS
Included in the pre-numbered kits are swabs for obtaining specimens from the penile-meatal orifice or vaginal swab with option for additional rectal swab. Detailed instructions for collection are included. Website users request the kits online, then log in after receiving notice by text or email that results are ready. Clinic options for obtaining treatment are listed on the website.
Study participants were of any gender, over 14 years of age, from Maryland and Washington D.C. who visited the IWTK website, and who wished to participate in the survey. The survey was voluntary, anonymous, and no questions were mandatory. The survey was open from November 28, 2017 to February 9, 2019. The design, including details of the survey, was determined by the Johns Hopkins University Johns Hopkins Institutional Review Board (IRB) to be “Exempt from Human Research” and was a Quality Improvement Project, (IRB 00126431).
Survey
For the purposes of this project, users of IWTK in Maryland and Washington D.C. areas were given an opportunity to complete a 16-question customer survey. Participants were asked for gender identity, age and ethnicity, and place of residence. They were then asked about prior use of the service. If they were prior users, this opened questions about their experience regarding ordering of the kit, experience overall, using the swabs, and instructions given. Further questions assessed the acceptability of at-home collection for samples for STI testing, and whether in-home rapid POC testing for STIs would be attractive, and the cost they would be willing to pay for such technology. In this analysis and report, only survey results were included for analysis. These anonymous data were not linked to test results or demographic data other than that described here.
Items related to user acceptability with IWTK were asked only of those who indicated that they had prior experience using the kit. Differences between genders and preference for the various sampling methods were analyzed with Pearson’s Χ2 test for univariate categorical data for significance, without Yates correction. P-values of ≤ 0.05 were considered significant. In general, non-responses were not included in the denominators for calculation, unless explicitly noted.
RESULTS
Data from the 457 respondents who completed any survey questions were included in this analysis. Demographics are in Table 1. All user survey questions had over a 93% response rate, except for the ease of using the collection swab, with a response rate of 93%.
Table 1. Baseline characteristics of survey participants (N=457).
Unless indicated otherwise, data are given as n/N where n is the number of respondents selecting a particular response, and N is the total number in the cohort.
| Demographic | n/N | % |
|---|---|---|
| Gender | ||
| Male | 168/457 | 36.8% |
| Female | 284/457 | 62.1% |
| Genderqueer | 3/457 | 0.7% |
| Trans male | 1/457 | 0.2% |
| No response | 1/457 | 0.2% |
| Place of Residence | ||
| Washington D.C. | 39/457 | 12.8% |
| Baltimore Met. Area | 112/457 | 36.8% |
| Other Maryland | 153/457 | 50.3% |
| No response | 4/457 | 0.9% |
| Race | ||
| American Indian/Alaska Native | 1/457 | 0.3% |
| Asian | 19/457 | 4.2% |
| Black | 166/457 | 36.3% |
| Multiracial | 53/457 | 11.6% |
| Unknown | 13/457 | 2.8% |
| White | 203/457 | 44.4% |
| No response | 2/457 | 0.4% |
| Age | ||
| Female | 284 | |
| 14-19 | 59/284 | 20.1% |
| 20-24 | 77/284 | 27.1% |
| 25-29 | 57/284 | 20.1% |
| 30-39 | 61/284 | 21.5% |
| 40-49 | 22/284 | 7.8% |
| 50+ | 8/284 | 2.8% |
| Male | 168 | |
| 14-19 | 16/168 | 9.5% |
| 20-24 | 32/168 | 19.1% |
| 25-29 | 30/168 | 17.9% |
| 30-39 | 56/168 | 33.3% |
| 40-49 | 22/168 | 13.1% |
| 50+ | 12/168 | 7.1% |
How Participants Learned of IWTK
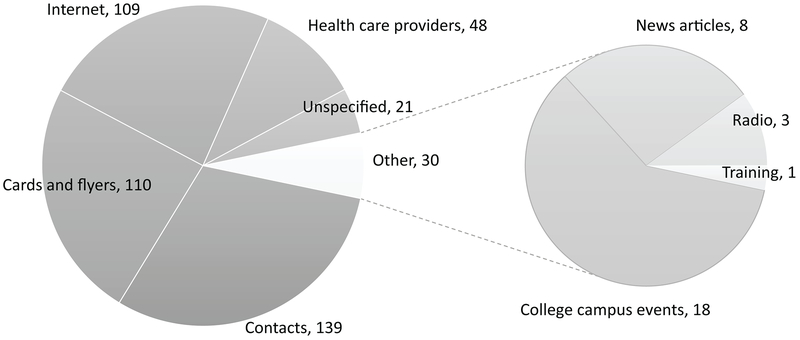
Results of this survey question were categorized into six general types of responses: personal contacts, internet, promotional materials, health care providers, and other (Figure 1). The largest group of respondents learned about IWTK from word of mouth contacts: family, friends, previous user, and partners (139/457 or 30%). Other common ways of learning about the program were the internet: Facebook, search engines, or unspecified internet (109/457 or 24%), IWTK promotion materials: cards or flyers (110/457 or 26%) and health departments and clinics (48/457 or 11%). Other ways mentioned (30/457 or 7%) were news articles, college campus events like safe sex carnivals, and hearing it mentioned on the radio. These results are shown in Figure 1. The proportion of users finding out about the program from the internet was similar among users of ages less than 40 (23% or 90/393) and those aged 40 and above (30% or 19/64).
Figure 1:
Responses to: How did you learn about IWTK? Numbers represent number of respondents selecting each response (N=457)
Prior experience with IWTK
There were 149 repeat users of IWTK in our sample (149/457 or 33%) with repeat users most often falling in the 30-39 age range category. The repeat users of IWTK were asked for their prior experience with IWTK on four aspects: ease of ordering a kit, rating their past experience, ease of using the collection swab, and ease of understanding the instructions (Table 2). The past users gave high marks on all of the aspects queried. For the question “how would you rate your past testing experience,” 138/144 or 96% of users answering this question selected “satisfied” or “very satisfied”.
Table 2. Previous User Experience with IWTK (N=149).
Previous users of the IWTK were asked the following questions:
How would you rate the ease of ordering a kit online?
How would you rate your past testing experience?
How would you rate the ease of using the collection swab?
How would you rate being able to understand the collection instructions?
| N | % | |
|---|---|---|
| Ease of Ordering | ||
| Easy | 18 | 12.8% |
| Very easy | 127 | 85.2% |
| No response | 4 | 2.7% |
| Rate Past Experience | ||
| Not satisfied | 1 | 0.7% |
| Neutral | 5 | 3.4% |
| Satisfied | 26 | 17.5% |
| Very Satisfied | 112 | 75.2% |
| No response | 5 | 3.4% |
| Swab Ease of Use | ||
| Neutral | 3 | 2.0% |
| Easy | 24 | 16.1% |
| Very easy | 112 | 75.2% |
| No response | 10 | 6.7% |
| Understand Collection Instructions | ||
| Neutral | 1 | 0.7% |
| Easy | 27 | 18.1% |
| Very easy | 116 | 77.9% |
| No response | 5 | 3.4% |
Home Testing for STIs
Our participants showed strong support for out of clinic screening. Overall 283/457 (62%) respondents stated home was their preferred place for testing for STIs, followed by 119/457 (26%) stating no preference, and only 26/457 (6%) preferring testing with a health care provider. There was no association between preferred location of testing based on age, gender, or by race.
Respondents were asked which specimens they prefer for self-collection, and allowed to choose among vaginal, penile, rectal, throat and urine collection, and able to mark multiple selections. Results from this question are showing in Table 3. In general, respondents preferred genital self-swab collection to self-collection of samples from extra genital sites. More females marked a preference for self-collection vaginal swabs over urine samples, (268/284 or 94%) of females preferring to self-collect vaginal swabs versus 154/284 or 54% selecting urine) and more males (126/168 or 75% of males) marked a preference for penile swabs over urine collection (110/168 or 64%). The gender preference for genital swabs was significant between male and female, with females more strongly preferring the genital swabs (p<0.01).
Table 3. Preference for self-collection swab locations by gender (N=452).
Data shown for the question: Which type of specimen would you prefer to self-collect for STI testing? (May select more than one response) Vaginal, Penile, Urine, Rectal swab, Throat Swab.
Numbers indicate number of users selecting preference for swab location by gender. Data are given as n/N where n is the number of respondents selecting a particular response, and N is the total number in the cohort, P values are with respect to gender differences.
| n/N | % | P | |
|---|---|---|---|
| Genital swabs | |||
| Female (vaginal) | 268/284 | 94% | <0.01 |
| Male (penile) | 126/168 | 75% | |
| Urine collection | |||
| Female | 154/284 | 54% | <0.01 |
| Male | 110/168 | 64% | |
| Rectal swabs | |||
| Female | 56/284 | 20% | <0.05 |
| Male | 61/168 | 36% | |
| Throat swabs | |||
| Female | 128/284 | 45% | 0.83 |
| Male | 74/168 | 44% |
Self-collected throat swabs were less popular than either of the above at 45% (205/457) of the respondents, and rectal swabs were the least selected for self-collection at 20% of females (56/284) and 36% of males (61/168), with a statistical difference between genders (p<0.01).
In-Home Point of Care Testing (POCT)
Respondents were asked how much they would be willing to pay for a hypothetical in-home rapid test device to detect STI if it were available. Users chose from options $10, $20, $30, $40, or $50+. Counting all respondents, the average cost willing to pay was $23.10, with $50+ assigned to be $50, which may undercount the true average. A strong majority (95% or 433/457) said they would be interested in a point-of-care rapid test if one were developed.
Participants were asked which STIs should a POCT be developed for, and a majority of respondents were in favor of such a test being developed for all of the STIs presented: (234/451 respondents answering this question voted for all of the listed STIs to have a home testing option, or 52%). Individually, the STI getting the most positive responses for development of at home POCT was chlamydia with 406 votes, followed by gonorrhea (384 votes), HSV (335 votes), trichomonas (334 votes), syphilis (327 votes), HIV (314 votes), HPV (300 votes), and hepatitis B and C, which were combined for this question (276 votes).
Most participants were willing to provide a fingerstick sample for the hypothetical syphilis kit, with 80% (364/457) indicating that they would be willing to self-collect the finger stick blood sample for a dried blood spot sample, 11% (48/457) were not sure, and only 9% (42/457) selecting that they would not be willing to provide this.
DISCUSSION
From survey responses from prior users of IWTK, respondents were quite satisfied by their previous experience, and found the swabs easy to use. Users were most interested in self-collection of vaginal or penile swabs, with throat and rectal swabs less popular for self-collection, with rectal swabs being least popular among female respondents for home self-collection.
There was also strong interest in pushing the technology further, to develop point-of-care testing options for STIs, with most respondents interested in home testing options for a range of STIs. The majority of participants would be willing to provide a fingerstick blood sample for such a kit. This is in line with other studies showing a high interest in such technology, as long as it provides high sensitivity for disease.2 A limitation of the study is that these results cannot be generalized to the broader population as our cohort was made of self-selected users of IWTK, a unique population already accepting of the idea of home collection for testing of STIs.
The self-collection kit for STI detection is a novel way to expand testing options for patients. It has high acceptability among users, and there is some research that suggests that self-collection may be a cost-effective option as well.3 Home collection for gonorrhea and chlamydia testing is currently offered by over a dozen private companies, Planned Parenthood in a few western states, and I Want the Kit in Maryland, Washington D.C. and Alaska.4 In this era of declining public health spending in the U.S. and declining availability for STI testing,5 it will be important to use the new opportunities that technology brings to expand access for patients, allowing them confidential, reliable, and affordable testing options.
Acknowledgments
Conflict of Interest and Sources of Funding: Funding through U54EB007958, NIBIB, NIH; U-01068613, NIAID, NIH grants. The authors declare no conflicts of interest.
REFERENCES
- 1.Gaydos CA, Dwyer K, Barnes M, et al. Internet-based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sex Transm Dis. 33:451–7, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Rompalo AM, Hsieh YH, Hogan T, et al. Point-of-care tests for sexually transmissible infections: What do ‘end users’ want? Sex Health. 2013;10(6):541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W, Gaydos CA, Barnes MR, et al. Cost-effectiveness analysis of Chlamydia trachomatis screening via internet-based self-collected swabs compared with clinic-based sample collection. Sex Transm Dis. 2011;38(9):815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haelle T You can order a dozen STD tests online - but should you? National Public Radio. August 13 2017. Available from: https://www.npr.org/sections/health-shots/2017/08/13/536905120/you-can-order-a-dozen-std-tests-online-but-should-you. [Verified 3 March 2019].
- 5.Leichliter JS, Heyer K, Peterman TA, et al. US public sexually transmitted disease clinical services in an era of declining public health funding: 2013-14. Sex Transm Dis. 2017;44(8):505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]