Abstract
Background and Purpose:
Injury to the corticospinal tract (CST) has been shown to have a major effect on upper extremity motor recovery after stroke. This study aimed to examine how well CST injury, measured from neuroimaging acquired during the acute stroke workup, predicts upper extremity motor recovery.
Methods:
Patients with upper extremity weakness after ischemic stroke were assessed using the upper extremity Fugl-Meyer (FM) during the acute stroke hospitalization and again at 3-month follow-up. CST injury was quantified and compared, using four different methods, from images obtained as part of the stroke standard-of-care workup. Logistic and linear regression were performed using CST injury to predict ΔFM. Injury to primary motor and premotor cortices were included as potential modifiers of the effect of CST injury on recovery.
Results:
N = 48 patients were enrolled 4.2 ± 2.7 days post-stroke and completed 3-month follow-up (median 90-day modified Rankin Scale 3, IQR 1.5). CST injury distinguished patients who reached their recovery potential (as predicted from initial impairment) from those who did not, with area under the curve (AUC) values ranging from 0.70 to 0.8. In addition, CST injury explained ~20% of the variance in the magnitude of upper extremity recovery, even after controlling for the severity of initial impairment. Results were consistent when comparing four different methods of measuring CST injury. Extent of injury to primary motor and premotor cortices did not significantly influence the predictive value that CST injury had for recovery.
Conclusions:
Structural injury to the CST, as estimated from standard-of-care imaging available during the acute stroke hospitalization, is a robust way to distinguish patients who achieve their predicted recovery potential and explains a significant amount of the variance in post-stroke upper extremity motor recovery.
Keywords: Recovery, Rehabilitation, Stroke Motor Recovery, Neurorehabilitation, Corticospinal Tract, Neuroimaging
SUBJECT TERMS: Ischemic Stroke, Imaging
INTRODUCTION
Upper extremity motor impairment is the most common source of disability after stroke,1 with the majority of spontaneous recovery occurring during the first three months.2, 3 Prior studies have shown that for most patients, the magnitude of arm motor recovery is directly proportional to the severity of initial impairment.4–6 Currently, however, even experienced clinicians find it difficult to predict recovery.7 Being able to accurately predict recovery early after stroke has the potential to inform patient-specific recovery potential, guide rehabilitation strategies including discharge destination, and stratify patients into recovery-focused clinical trials.8–10
The corticospinal tract (CST) is an important pathway for voluntary dexterous upper extremity movement as well as for upper extremity motor recovery.11 Structural and functional measures of integrity of the CST—specifically the presence of motor evoked potentials (MEPs) induced by transcranial magnetic stimulation (TMS)12 and fractional anisotropy values derived from diffusion tensor imaging (DTI)13, 14—have been used to predict motor recovery after stroke15, 16, but these methods require dedicated research imaging methods17 and are currently not widely clinically available equipment.18 Methods that use common clinical imaging19, without the necessity for dedicated additional scanning or specific equipment, have the substantial potential benefit of being readily and widely applicable early after stroke, across different clinical sites, and large numbers of patients.
Prior studies that have calculated structural injury to the CST using clinically available imaging after stroke have employed heterogeneous methods.19–23 The general approach requires a CST template, individual stroke lesion maps, and a method for calculating the overlap between the two, but the templates used and methods of overlap vary substantially. No study has yet systematically compared different methods for estimating CST injury from acute stroke imaging with the goal of predicting spontaneous upper extremity motor recovery. Analysis of clinically available imaging also enables quantification of injury to other brain areas beyond the CST known to be important for motor recovery after stroke, for example the primary motor cortex (M1) and premotor cortex (PM).21, 24–26 Studies to date have not yet quantified the relative contribution of CST, PM and M1, and the interactions among them to predict spontaneous or therapy-related motor recovery after stroke.
The primary aim of this study was to examine how well CST injury predicts upper extremity motor recovery using neuroimaging acquired during the acute stroke workup. Our hypothesis was that CST injury estimated from acute stroke images has significant predictive value for upper extremity motor recovery. Secondary aims included (1) comparing prediction performance of four different CST injury methods, and (2) assessing the effect of PM and M1 injury on arm recovery.
METHODS
The data and analysis code that support the findings of this study are available from the corresponding author upon reasonable request.
Subject Recruitment
Patients were recruited from an ongoing, prospective, single-center natural history study of recovery of upper extremity weakness after stroke, the Stroke Motor Recovery and Rehabilitation Study (SMaHRT, http://www.clinicaltrials.gov, ). From June 2017 to December 2018, eligible stroke patients with upper extremity weakness were screened from the Massachusetts General Hospital inpatient stroke service (detailed inclusion/exclusion in Supplemental Methods). All participants in the study provided written informed consent. The Institutional Review Board at Partners Healthcare approved the study.
Research Testing and Outcome Measures
Baseline information on age, gender, ethnicity, handedness, affected arm, premorbid disability, NIH stroke scale (NIHSS), prior stroke, treatment status with respect to tissue plasminogen activator (tPA) or endovascular therapy (IA therapy), and infarct location were recorded for all participants at the time of enrollment. At follow-up, the presence of post-stroke depression (defined by Patient Health Questionnaire score > 427), whether patients were taking anti-depressants (fluoxetine, other selective serotonin reuptake inhibitor, SSRI, or other non-SSRI antidepressant), and 90-day modified Rankin Scale (mRS) were recorded. Participants were assessed with standard rehabilitation outcome measures including the upper extremity Fugl-Meyer (FM)28, 29 during the acute stroke hospitalization (FMinit) and again at 3-month follow-up (FM3mo). Trained occupational therapists and researchers performed the upper-extremity FM evaluation, a reliable and validated measure of motor impairment after stroke. Assessors who collected outcome measures were not involved in treating patients. Each patient participated in standard therapy between study enrollment and 3-month follow-up.
Image Processing and Lesion Mapping
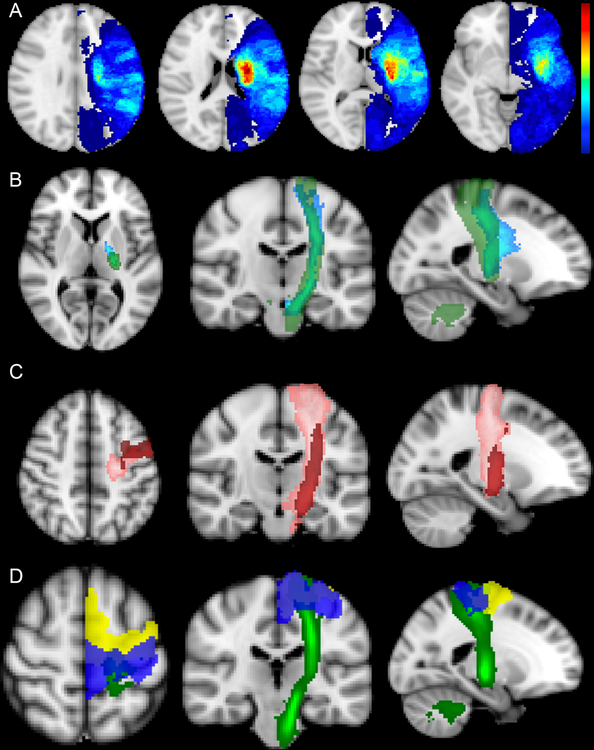
Stroke topography was determined using MRI DWI images obtained as part of the standard-of-care acute stroke inpatient workup. In the case of multiple MRIs performed during the acute stroke hospitalization, preference was given to scans closest to the inpatient research assessment date. In one case, MRI imaging was clinically contra-indicated and the patient’s CT scan was used. Methods for normalizing brain images to templates based on mixed CT and MRI modalities are well-established.30 Lesions were manually traced on DWI/ADC volumes using FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) by research staff (AC and FG) and independently verified by two board-certified neurologists (DJL and SBS). This manual segmentation process takes approximately 20–30 minutes to perform depending on the size and complexity of the lesion, plus a few minutes to run scripts for spatial transformation and metric extraction (see Supplemental Methods for further details). Figure 1a shows the lesion overlap maps for the participants in this study.
Figure 1.
(A) Stroke lesion overlap maps for the 48 participants. All lesions were flipped onto the left hemisphere for display. Colorbar on the right with maximum value 22 (i.e. maximal overlap voxel, red). (B) Primary motor cortex (M1) - Corticospinal tract (CST) templates constructed using deterministic tractography methods. The light blue tract shows an M1-CST from 17 healthy controls at University of California at Irvine (UCI). The green tract shows an M1-CST from 28 health subjects at Johns Hopkins University (JHU). Note the tracts are slightly offset and that the JHU tract traverses down to the level of the medulla while the UCI tract stops at the level of the mid-pons. (C) Templates of dorsal premotor (PMd, pink) and ventral premotor (PMv, red) CSTs. (D) Templates of primary motor (M1, dark blue), and premotor (pM), yellow) cortices overlaid on the JHU CST template (green).
Corticospinal Tract Injury
Two separate canonical M1-CST (unilateral M1 seed region) templates were used in this study (Figure 1b). One was generated at University of California Irvine (UCI) with 17 healthy, right-handed subjects using diffusion-weighted images obtained at 3T as previously described.24 The other M1-CST tract was from the Johns Hopkins University (JHU) white-matter tractography atlas,31 which averages the deterministic tractography of 28 healthy subjects and is available as part of the FSL software package. Note that the JHU tract traverses down to the level of the medulla while the caudal aspect of the UCI tract ends at the mid-pons. CST tracts from unilateral premotor seed regions, specifically dorsal premotor (PMd) and ventral premotor (PMv) cortices, were also included (Figure 1c). PMd- and PMv- CST tracts were generated from 12 healthy right-handed subjects with tractographic methods for generating probability pathways connecting PMd and PMv to cerebral peduncle as described previously24.
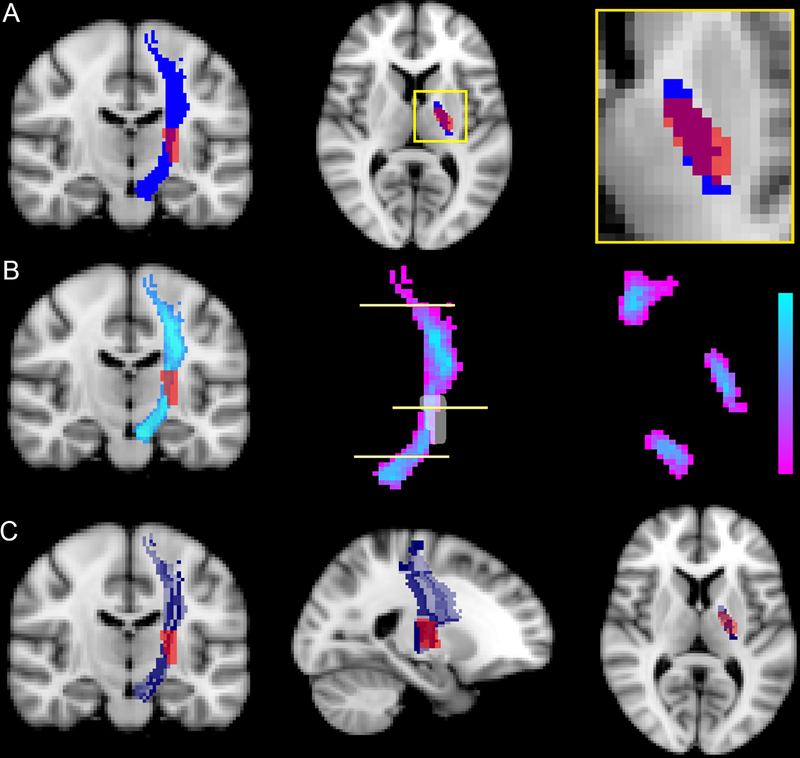
For each CST template, four different and previously published methods for estimating CST injury were implemented (Figure 2). In the first method, CST injury was calculated on the transverse slice of the CST with greatest lesion overlap.21 In the second and third methods, raw- and weighted- CST lesion loads, respectively, were calculated as previously described.23 In the final method, each CST was divided into 16 longitudinal subsections aimed at modeling the trajectory of groups of axons. The extent of injury to each subsection was quantified by measuring the volume of overlap between that subsection and the stroke mask. We then used the previously applied threshold of 5% overlap to designate the subsection injured by the stroke and, furthermore, determined the percentage of subsections injured by the stroke.24 All CST injury methods were implemented in MATLAB (Mathworks Inc, Natick, MA).
Figure 2.
Different methods for estimating CST injury. There are a variety of methods for calculating CST injury from a given stroke lesion and CST template. (A) One method uses area overlap between the stroke lesion and binarized CST tract on the axial slice with maximal overlap. Left panel shows coronal binarized CST tract, middle shows axial slice with maximal overlap between stroke and CST, right shows zoomed overlap of stroke (red) and CST template (blue). The # of voxels overlapped (red-blue overlay) are divided by the total # of blue voxels. (B) In calculating 3D volume overlap between stroke (red) and CST (blue), raw- and weighted- values are incorporated into CST weighting to account for the probabilistic nature of the CST and narrowing of the CST at different points (i.e. the posterior limb of the internal capsule). Left panel shows probabilistic nature of CST in a coronal slice. Middle panel highlights the weighted nature of the tract with purple to light blue colorbar. The horizontal lines indicate corresponding axial slices of the CST that are shown in the right panel. (C) Another method divides the CST into a number of rostral-caudal strands (16 in this case) and calculates % injury to each strand. If any given strand is lesioned by more than 5%, the strand is classified as injured. The right, middle, and left panels show coronal, sagittal, and axial slices respectively with CST strands in different colors (gray-blue gradient) and with stroke lesion shown in red.
Injury to Primary Motor and Premotor Cortices
Templates of the primary motor cortex (M1) and premotor cortex (PM), which includes dorsal and ventral premotor cortices (PMd and PMv), (Figure 1d) were obtained from the Julich Histological Atlas32 available as part of the FSL software package. These templates were binarized. Injury to M1 and PM were quantified by calculating the volume overlap between the respective binarized template and stroke masks. Given that M1 and PM injury were tightly correlated (Pearson’s R = 0.9), M1 and PM were treated as a single region-of-interest, M1-PM.
Statistical Analysis
Recovery was defined as change in FM between initial testing and 3-month follow-up:
The potential for recovery was the difference between initial FM and the maximum possible FM score:
Methods for distinguishing proportional from limited recoverers have previously been described4–6 and are detailed in Supplemental Methods.
The Kolmogorov–Smirnov test (KS test) was used for unadjusted comparison of the performance of different CST injury methods in their ability to distinguish proportional from limited recoverers. Adjusted comparison controlled for lesion volume using multivariate logistic regression. To understand the effect of clinical characteristics including age, handedness, affected arm, depression, antidepressant use, premorbid disability, acute stroke treatment, prior stroke, stroke location, and subscales of the NIHSS (aphasia, neglect, sensory deficit) on recoverer status (proportional vs. limited recovery), KS, Fisher’s exact, and chi-squared tests were performed for continuous and ordinal, dichotomous, and nominal values respectively.
To further understand the ability of different CST injury methods to explain recovery, each individual’s FM change was normalized by their potential for recovery to determine their realized recovery:
This measure varies from 0 to 1 and accounts for initial injury. The rationale for this normalization is that an individual who starts at an FMInit of 58 and recovers to 64 at 3 months would be hypothesized to have a different (i.e. much smaller burden of) CST injury from an individual who starts at an FMInit of 4 and recovers to 10. Note that normalizing recovery in this way accounts for the proportional recovery model assumption that initial injury explains the vast amount of variance in change. We performed Pearson’s correlation and calculated the R2 between values for each CST injury method and realized FM recovery.
Finally, to understand the influence of cortical injury on the relationship between CST injury and recovery, hierarchical linear regression including CST injury, M1-pM injury, and the interaction term was performed. Statistical analyses were performed in MATLAB (Mathworks, Natick, MA).
RESULTS
Study Subjects
A total of 65 patients with upper extremity weakness after ischemic stroke consented for this study and had initial research measures collected during their acute stroke hospitalization. N = 48 participants completed their 90-day research follow-up at the time of this analysis. Reasons for not completing the 90-day research follow up included death (4), recurrent stroke (4), lost to follow-up (6), study withdrawal (1), and inability to complete follow-up research assessments because of time (1) and pain (1). Participants were assessed within 4.2 ± 2.7 (mean ± standard deviation) days and again 85.4 ± 7.3 days after stroke onset. Baseline and stroke characteristics are summarized in Table 1 and provided for individual participants in Supplemental Table I.
Table 1.
Participant characteristics. Statistics are presented as mean ± ste, n (%), and median [IQR]. Kolmogorov-Smirnov, Fisher’s exact, and chi-squared tests were performed for continuous (age, total NIHSS) and ordinal (mRS), dichotomous (gender, dominant hand, affected arm, depression, prior stroke, acute stroke therapies, and presence of aphasia, neglect, or sensory deficit), and nominal (ethnicity, antidepressant use, and stroke location) values, respectively.
| Total Sample n = 48 |
Proportional Recoverers n = 31 |
Limited Recoverers n = 17 |
p-value | |
|---|---|---|---|---|
| Age | 64.81 ± 1.67 | 65.61 ± 1.59 | 63.35 ± 3.77 | 0.73 |
| Gender (Male) | 25 (52.1) | 19 (61.3) | 6 (35.3) | 0.13 |
| Ethnicity | χ2 = 0.60 p = 0.74 |
|||
| White | 42 (87.5) | 27 (87.1) | 15 (88.2) | |
| Black | 5 (10.4) | 3 (9.7) | 2 (11.8) | |
| Hispanic | 1 (2.1) | 1 (3.2) | 0 | |
| Dominant Hand (Right) | 39 (81.3) | 27 (87.1) | 12 (70.6) | 0.25 |
| Affected Arm (Right) | 23 (47.9) | 17 (54.8) | 6 (35.3) | 0.24 |
| Depression (Follow-up) | 13 (27.1) | 7 (22.6) | 6 (35.3) | 0.5 |
| Antidepressant Use | χ2 = 4.34 p = 0.23 |
|||
| Fluoxetine | 28 (58.3) | 15 (48.4) | 13 (76.5) | |
| Other SSRI | 5 (10.4) | 4 (12.9) | 1 (5.9) | |
| Non-SSRI | 6 (12.5) | 4 (12.9) | 2 (11.8) | |
| Premorbid mRS* | 0 [1] | 0 [0.75] | 0 [2] | 0.14 |
| Prior Stroke | 9 (18.8) | 7 (22.6) | 2 (11.8) | 0.46 |
| Acute Stroke Therapy | ||||
| tPA+ | 16 (33.3) | 12 (38.7) | 4 (23.5) | 0.35 |
| EVT‡ | 10 (20.8) | 6 (19.4) | 4 (23.5) | 0.73 |
| Stroke Location | χ2 = 1.84 P = 0.61 |
|||
| MCA | 37 (77.1) | 25 (80.7) | 12 (70.6) | |
| PCA | 2 (4.2) | 1 (3.2) | 1 (5.8) | |
| Brainstem | 4 (8.3) | 3 (9.7) | 1 (5.8) | |
| Multifocal | 5 (10.4) | 2 (6.5) | 3 (17.7) | |
| Initial NIHSS | 7.5 [7] | 5 [3.75] | 13 [7.25] | < 0.001 |
| Aphasia | 10 (20.8) | 7 (22.6) | 3 (17.7) | 1 |
| Neglect | 13 (27.1) | 5 (16.1) | 8 (47.1) | 0.46 |
| Sensory Deficit | 21 (43.8) | 12 (38.7) | 9 (52.9) | 0.34 |
| Initial Fugl-Meyer | 20.5 (48.5) | 44 (34) | 4 (3.5) | < 0.001 |
| Fugl-Meyer 3 months | 58 (46.5) | 63 (7.5) | 9 (14.25) | < 0.001 |
| 90-day mRS | 3 (1.5) | 2 (2) | 4 (1) | < 0.001 |
mRS = modified Rankin Scale
tPA = tissue plasminogen activator
EVT = endovascular therapy
CST Injury Methods Distinguish Proportional Spontaneous Recoverers from Limited Recoverers
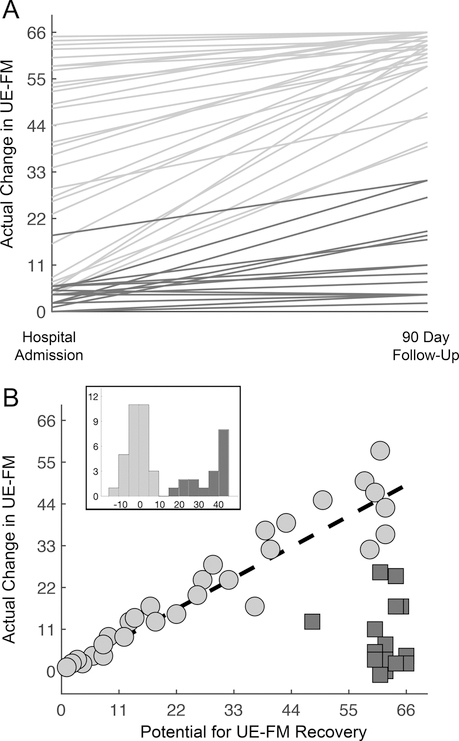
There were 31 proportional recoverers and 17 limited recoverers (Figure 3). The group of limited recoverers on averaged started with initially severe arm impairment (FM < 22) and experienced a median of 10% of their potential for FM recovery, in contrast to the group of proportional recoverers who on average started with FM >= 22 and realized a median of 86% of their arm recovery potential (p < 0.001). Four methods (max area overlap, raw lesion-load, weighted lesion-load, and 16-Div, 5% injury) for estimating CST injury from neuroimaging acquired during the acute stroke workup were compared for their ability to distinguish proportional recoverers from limited recoverers. First, there was high correlation between the four methods’ injury values, across all canonical CST tracts (UCI M1-CST, JHU M1-CST, PMd-CST, PMv-CST) (Supplemental Table II). CST injury, regardless of method, tract, or seed region used, separated proportional from limited recoverers. This was true whether using unadjusted models or adjusted models that incorporated lesion volume (Table 2). Receiver operating characteristic (ROC) analysis (Supplemental Figure II and Supplemental Table III) showed that area under the curve (AUC) values for all four CST injury methods for distinguishing limited from proportional recoverers ranged from 0.70 to 0.8, indicating good discrimination ability.33 Note that AUC values for M1-CST tracts showed slightly better discriminatory ability (0.75 – 0.8, Supplemental Table III top) than AUC values for PMd-CST and PMv-CST tracts (0.70 – 0.75, Supplemental Table III bottom). In contrast to all CST injury methods, clinical characteristics including post-stroke depression, antidepressant use, presence of prior stroke, and presence of aphasia or neglect were not significantly related to recoverer-status (Table 1).
Figure 3.
Proportional and limited recoverers. (A) Upper extremity Fugl-Meyer (FM) recovery curves between hospital admission and 3-month follow-up for 48 patients with stroke. Note that in severe patients (FMInit < 22) there is a group with limited recovery (dark gray lines). (B) Potential for (66 – FMInit) versus actual (FM3mo – FMInit) recovery of upper extremity impairment. The line (black-dashed) represents the amount of recovery as predicted by the proportional recovery model (FMPredicted = 0.7 × FMPotential). Limited recoverers (dark gray squares) are distinguished from proportional recoverers by a model residual of greater than 10 from their 70% predicted recovery. The histogram inset shows the model residuals of proportional recoverers (light gray) and limited recoverers (dark gray).
Table 2.
Performance of different CST injury methods for distinguishing proportional from limited recoverers. Values are presented as mean ± se. Abbreviations for different CST injury methods: Max Area = maximum cross-sectional area, RLL = raw lesion load, WLL = weighted lesion load, 16/5% = 16 divisions, 5% injury threshold
| CST Template | Injury Method | CST Injury Value | p (KS test)+ |
p (adjusted) ‡ |
|
|---|---|---|---|---|---|
| Proportional (n=31)* |
Limited (n=17)* |
||||
| UCI, M1 | Max Area | 0.47 ± 0.061 | 0.75 ± 0.060 | 0.0080 | 0.017 |
| UCI, M1 | RLL (cc) | 0.86 ± 0.14 | 2.0 ± 0.35 | 0.0034 | 0.0042 |
| UCI, M1 | WLL (cc) | 1.42 ± 0.22 | 3.26 ± 0.56 | 0.0037 | 0.0058 |
| UCI, M1 | 16/5% | 0.44 ± 0.063 | 0.75 ± 0.050 | 0.0067 | 0.013 |
| JHU, M1 | Max Area | 0.36 ± 0.044 | 0.64 ± 0.061 | <0.001 | 0.0022 |
| JHU, M1 | RLL (cc) | 0.26 ± 0.04 | 0.68 ± 0.12 | <0.001 | 0.0037 |
| JHU, M1 | WLL (cc) | 0.50 ± 0.08 | 1.23 ± 0.21 | 0.0011 | 0.0037 |
| JHU, M1 | 16/5% | 0.21 ± 0.038 | 0.47 ± 0.080 | 0.0047 | 0.0056 |
| PMd | Max Area | 0.44 ± 0.048 | 0.66 ± 0.060 | 0.0070 | 0.021 |
| PMd | RLL (cc) | 1.11 ± 0.18 | 2.52 ± 0.48 | 0.012 | 0.0034 |
| PMd | WLL (cc) | 1.77 ± 0.27 | 4.02 ± 0.69 | 0.0022 | 0.0020 |
| PMd | 16/5% | 0.39 ± 0.057 | 0.65 ± 0.084 | 0.015 | 0.022 |
| PMv | Max Area | 0.42 ± 0.060 | 0.68 ± 0.079 | 0.012 | 0.026 |
| PMv | RLL (cc) | 0.64 ± 0.13 | 1.51 ± 0.36 | 0.017 | 0.0088 |
| PMv | WLL (cc) | 1.18 ± 0.23 | 2.90 ± 0.64 | 0.0053 | 0.0031 |
| PMv | 16/5% | 0.37 ± 0.060 | 0.63 ± 0.085 | 0.025 | 0.026 |
| Lesion Volume (cc) | 35.0 ± 10.3 | 53. 6 ± 16.2 | 0.14 | - | |
Note that UCI template calculations could not be performed on 5/48 (3 proportional and 2 limited recoverers) because these participants had injury that extended below the level of the mid-pons. JHU template calculations were performed on all 48 participants.
KS test = Kolmogorov-Smirnov test.
logistic regression adjusted for lesion volume.
CST Injury Explains ~20% of Individual Variance in Spontaneous Motor Recovery
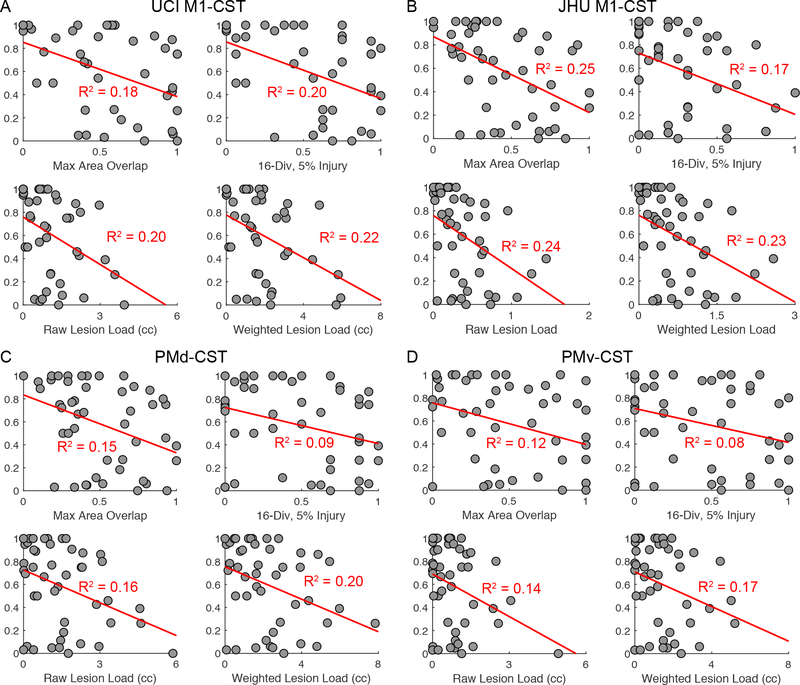
Next we sought to extend these findings regarding proportional versus limited recovery to understanding how CST injury explains variance in individual recovery. Realized recovery (a measure of recovery adjusted for extent of possible recovery) was examined in relation to CST injury values generated using each of the CST injury methods. M1-CST injury, across each method and both canonical M1-CST tracts, was related to realized recovery across all subjects with R2 values ranging from 0.18 to 0.25 (Figure 4 A,B, all p < 0.01). PMd- and PMv- CST injury, across each method, though still significant, had less strong relationships with realized recovery with R2 values ranging from 0.08 to 0.20 (Figure 4 C,D, all p < 0.05). Structural injury to the CST significantly explains ~20% of the variance in upper extremity motor recovery even after adjusting for initial motor deficits.
Figure 4.
Scatter plots of realized recovery against all different CST injury method values for the (A) UCI M1-CST, (B) JHU M1-CST (C) PMd-CST and (D) PMv-CST. Least-square fit lines and corresponding R2 correlation coefficients are shown in red.
Adding Measures of Motor and Premotor Cortex Injury Does Not Affect the Relationship between CST Injury and Recovery
A total of 21 patients sustained injury to M1-pM while 27 had no injury. In contrast to CST injury, total amount of injury to M1-pM did not separate proportional from limited recovery in both unadjusted (p = 0.54) and adjusted (P = 0.17) models. Total amount of M1-pM injury also did not significantly explain variance in realized recovery (R2 = 0.1, P = 0.08).
We chose the cross-sectional overlap method on the JHU tract as our primary CST injury method because it achieved the greatest R2 and could be performed in all patients in the cohort including those with stroke lesions below the level of the mid-pons. In a hierarchical linear regression analysis (Supplemental Table V), CST injury explained 24.8% of the variance in realized recovery (Model 1) and addition of M1-pM injury did not significantly change the R2 (Model 2). Model 3 included CST injury, M1-pM injury, and their interaction term to determine whether the presence of M1-pM injury moderated the relationship between CST and realized recovery. Although the slope of the interaction term was negative, suggesting that there was a trend toward M1-pM injury reinforcing the effect of CST injury on recovery, the interaction term was not significant. The results of Models 2 and 3 were unchanged whether we included amount of M1-pM injury as a binary or continuous variable. Furthermore, structural injury to M1-pM did not significantly moderate the relationship between CST injury and realized recovery.
DISCUSSION
The main findings in this study were (1) extent of CST injury distinguishes proportional recoverers from limited recoverers, (2) CST injury explained ~20% of variance in the magnitude of upper extremity recovery, even after adjusting for initial motor deficits, and (3) injury to primary motor and premotor cortices did not significantly influence the predictive value that CST injury had on arm motor recovery. Importantly, these motor system injury measures were derived from neuroimages acquired as part of the acute stroke standard evaluation. Together these findings suggest that acute imaging provides useful data for predicting behavioral recovery after stroke.
Predicting the extent to which a patient will recover after stroke is useful for informing clinical decision-making as well as for stratification in clinical trials.8–10 Some methods proposed for predicting recovery after stroke require specialized research equipment or imaging techniques that are accessible only in advanced research centers.34 The current study therefore examined prediction of recovery using scans acquired as part of standard of care. We found that CST injury distinguished patients who achieved their predicted recovery from those who did not, regardless of method implemented or tract used. CST injury also significantly explained ~20% of variance in the magnitude of individual recovery. This remained true after controlling for the severity of initial arm impairment.
Significant agreement was found between four different methods for estimating CST injury from acute stroke imaging, including raw- and weighted- lesion load. This suggests that these methods are relatively precise and that clinicians and investigators can likely pick their method and template of choice for estimating CST injury from acute stroke images. One prior study also employed a similar approach, using raw- and weighted- CST lesion load derived from acute stroke imaging to predict upper extremity motor impairment outcomes at 3 months, and found that weighted-lesion load performed best for prediction in their cohort19. CST templates from primary motor as compared to premotor cortex allowed for better recovery prediction suggesting that the specificity of the cortical seed region is important for CST-related motor recovery.
Structural injury to primary motor and premotor cortices themselves did not significantly influence the relationship between CST injury and recovery. The relationship, however, was in the expected direction — M1-PM injury reinforced the negative effect of CST injury on recovery. The lack of significance may have been a reflection of the sample size (n = 48) in this single-site study. Furthermore, standard template-based imaging assignments of cortical regions of interest may not precisely align with classical neuroanatomy or functional neurophysiology. To this end, the application of more recent approaches to cortical parcellation35 may prove useful for understanding recovery after stroke.
The current methods set the stage to employ clinical standard-of-care stroke imaging to predict a patient’s extent of recovery after stroke. Measuring injury to a single white matter tract using acute stroke imaging significantly predicts recovery, but significant variance remains unexplained—there were cases in which proportional recoverers had substantial CST injury and limited recoverers had modest injury, although separation between the two groups was overall good with ROC AUC values of 0.7 – 0.8. Adding measures of neural function, such as transcranial magnetic stimulation15, 16 or EEG36 may improve the strength of prediction but are not currently part of the standard of care acute stroke evaluation. Much as neuroimaging guides decision-making in the context of reperfusion therapy decision-making, the same images can inform decision-making in a recovery-focused context.
There are a number of limitations to this study. As compared to national statistics, our study sample had a slightly younger mean age (65 in this study compared to 69 nationally37), had slightly more men (52% in this study compared to 47% nationally38), and was highly ethnically homogeneous (White) reflecting the patient population at our single center. Only one motor outcome measure was used in this study, the upper extremity FM, which may not reflect the complexity of motor and premotor system function. Including measures of complex motor behaviors (such as apraxia) with specific relationships to cortical areas and descending tracts may improve predictive ability. Similarly, including early and more sophisticated (beyond NIHSS subscales) measures of co-factors that would be hypothesized to influence motor recovery such as cognition, aphasia, neglect, sensation, handedness, and dominant/non-dominant hemisphere of stroke injury would likely improve recovery prediction. Finally, estimating the topography of stroke injury from acute stroke diffusion images presents clear challenges. Our study relied on manual lesion segmentation, which is inherently subjective but currently remains the gold standard. In addition, there are a number of early stroke features that were not accounted for including post-stroke edema, which may influence the neuroanatomic localization of the stroke lesion relative to motor/premotor cortices or descending corticospinal tracts. Machine learning techniques applied to large datasets of stroke images and rehabilitation outcome measures may be helpful for both automating lesion segmentation and learning important imaging features39.
SUMMARY / CONCLUSIONS
Structural injury to the CST estimated from standard-of-care imaging is a robust way to distinguish patients who achieve their predicted recovery from those who do not, and explains a substantial amount of variance in post-stroke upper extremity motor recovery. These methods provide a scalable model for subsequent stroke recovery studies. Predictors of stroke recovery have the potential to inform targeted development of stroke recovery therapeutics, stratify patients in clinical trials, and personalize neurorehabilitation clinical decision-making.40
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Susan E. Fasoli for standardized upper extremity Fugl-Meyer training provided to research staff in this study and Amanda Duffy, Susan E. Fasoli and Teresa J. Kimberley for helpful comments provided to the manuscript.
SOURCES OF FUNDING
This research was supported by the NIH through R25NS065743 (Lin) and K99HD091375 (Cassidy), 2R44NS095381-02 (Finklestein), and the American Heart Association / Northeast Cerebrovascular Consortium pilot grant (Lin), and the Massachusetts General Hospital Center for Neurotechnology and Neurorecovery.
Footnotes
DISCLOSURES
Dr. Lin has served as a consultant for Boehringer Ingelheim. Dr. Finklestein is a Principal in Stemetix, and a Consultant to AZTherapies, Constant Therapeutics, Elevian, NeuroVasc, and Regenera. Dr. Cramer has served as a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, Regenera, SanBio, Stemedica, Fujifilm Toyama Chemical Co., Biogen, and TRCare. Dr. Hochberg has received funding from the AHA (19CSLOI34780000) for research unrelated to this project. Dr. Schwamm reports serving as chair of the AHA/ASA GWTG stroke clinical work group and hospital accreditation Science Committee and Quality Oversight Committees; and serving as a scientific consultant to LifeImage regarding user interface design and usability, and regarding trial design and conduct to Penumbra (data and safety monitoring committee, Separator 3D and MIND trials) and Medtronic (Victory AF and Stroke AF trials).
REFERENCES
- 1.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: A systematic review. Lancet Neurol 2009;8:741–754 [DOI] [PubMed] [Google Scholar]
- 2.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37:2348–2353 [DOI] [PubMed] [Google Scholar]
- 3.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089 [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71 [DOI] [PubMed] [Google Scholar]
- 5.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2015;29:614–622 [DOI] [PubMed] [Google Scholar]
- 6.Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. Proportional motor recovery after stroke: Implications for trial design. Stroke. 2017;48:795–798 [DOI] [PubMed] [Google Scholar]
- 7.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G, Early Prediction of Functional Outcome After Stroke I. Accuracy of physical therapists’ early predictions of upper-limb function in hospital stroke units: The epos study. Phys Ther 2013;93:460–469 [DOI] [PubMed] [Google Scholar]
- 8.Stinear CM, Byblow WD, Ackerley SJ, Barber PA, Smith MC. Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke. 2017;48:1011–1019 [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. 2017;12:444–450 [DOI] [PubMed] [Google Scholar]
- 10.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2017;31:864–876 [DOI] [PubMed] [Google Scholar]
- 11.Rondina JM, Park CH, Ward NS. Brain regions important for recovery after severe post-stroke upper limb paresis. J Neurol Neurosurg Psychiatry. 2017;88:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015;78:848–859 [DOI] [PubMed] [Google Scholar]
- 13.Bigourdan A, Munsch F, Coupe P, Guttmann CR, Sagnier S, Renou P, et al. Early fiber number ratio is a surrogate of corticospinal tract integrity and predicts motor recovery after stroke. Stroke. 2016;47:1053–1059 [DOI] [PubMed] [Google Scholar]
- 14.Buch ER, Rizk S, Nicolo P, Cohen LG, Schnider A, Guggisberg AG. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology. 2016;86:1924–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The prep algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135:2527–2535 [DOI] [PubMed] [Google Scholar]
- 16.Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. Prep2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017;4:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim B, Fisher BE, Schweighofer N, Leahy RM, Haldar JP, Choi S, et al. A comparison of seven different dti-derived estimates of corticospinal tract structural characteristics in chronic stroke survivors. J Neurosci Methods. 2018;304:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoonhorst MHJ, Nijland RHM, van den Berg PJS, Emmelot CH, Kollen BJ, Kwakkel G. Does transcranial magnetic stimulation have an added value to clinical assessment in predicting upper-limb function very early after severe stroke? Neurorehabil Neural Repair. 2018;32:682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol 2015;78:860–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassidy JM, Tran G, Quinlan EB, Cramer SC. Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke. 2018;49:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam TK, Binns MA, Honjo K, Dawson DR, Ross B, Stuss DT, et al. Variability in stroke motor outcome is explained by structural and functional integrity of the motor system. Sci Rep 2018;8:9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol 2015;77:132–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol 1998;55:1081–1088 [DOI] [PubMed] [Google Scholar]
- 26.Schulz R, Park E, Lee J, Chang WH, Lee A, Kim YH, et al. Interactions between the corticospinal tract and premotor-motor pathways for residual motor output after stroke. Stroke. 2017;48:2805–2811 [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31 [PubMed] [Google Scholar]
- 29.See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, et al. A standardized approach to the fugl-meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27:732–741 [DOI] [PubMed] [Google Scholar]
- 30.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific ct and mri templates for spatial normalization. Neuroimage 2012;61:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39:336–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 2007;36:511–521 [DOI] [PubMed] [Google Scholar]
- 33.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5:1315–1316 [DOI] [PubMed] [Google Scholar]
- 34.Kim B, Winstein C. Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair. 2017;31:3–24 [DOI] [PubMed] [Google Scholar]
- 35.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Srinivasan R, Burke Quinlan E, Solodkin A, Small SL, Cramer SC. Utility of eeg measures of brain function in patients with acute stroke. J Neurophysiol 2016;115:2399–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology 2012;79:1781–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 39.Liew SL, Anglin JM, Banks NW, Sondag M, Ito KL, Kim H, et al. A large, open source dataset of stroke anatomical brain images and manual lesion segmentations. Sci Data. 2018;5:180011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin DJ, Finklestein SP, Cramer SC. New directions in treatments targeting stroke recovery. Stroke. 2018;49:3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.