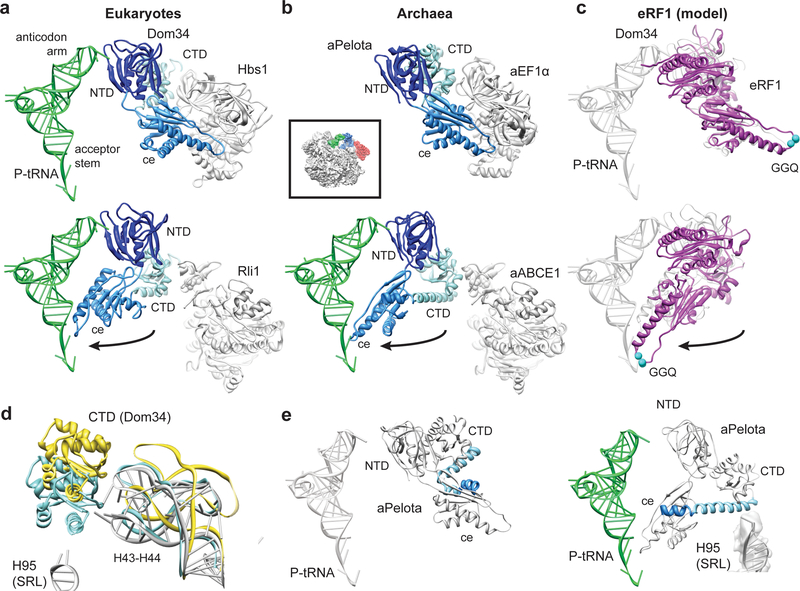
Fig. 3: Domain movements in Pelota and eRF1.
(a) Comparison of the ribosome-bound Dom34 conformation in complex with Hbs1 (top section) and Rli1 (lower section). (b) Comparison of the aPelota-aEF1α crystal structure32 with the ribosome-bound aPelota in complex with aABCE1. The central domain of Pelota swings out towards the P-site tRNA. (c) Models for eRF1 before and after the suggested movement of the central domain. (d) Conformation of the Dom34 CTD and the stalk base rRNA (H43-H44) when bound to Hbs1 (yellow) and to Rli1 (blue). rRNA conformation without factors bound is shown in grey. (e) In aPelota three separate small helices refold into a long α-helix during movement of the central domain bridging the CTD and the central domain.