SYNOPSIS
Autism spectrum disorder (ASD) emerges during early childhood and is marked by a relatively narrow window in which infants transition from exhibiting normative behavioral profiles to displaying the defining features of the ASD phenotype in toddlerhood. Prospective brain imaging studies in infants at high familial risk for autism have revealed important insights into the neurobiology and developmental unfolding of ASD, showing great promise for both presymptomatic detection and informing the timing and nature of early intervention. In this article, we review neuroimaging studies of brain development in ASD from birth through toddlerhood, relate these findings to candidate neurobiological mechanisms, and discuss implications for future research and translation to clinical practice.
Keywords: neurodevelopment, neuroimaging, psychoradiology, infant, brain, autism spectrum disorder, magnetic resonance imaging, diffusion tensor imaging
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder diagnosed in 1 in 59 children in the US1. ASD is characterized by heterogeneous symptom profiles associated with varying levels of severity in social communication deficits and restricted and repetitive behaviors. There has been considerable interest in understanding the neurobiology of ASD, with neuroimaging playing a key role in describing the neuroanatomy and physiology of individuals with ASD for over three decades. However, the vast majority of studies to date have occurred post-diagnosis and been cross-sectional in nature, collapsing across wide age ranges. Given that we now understand that brain development – and the development of ASD2–5 – is non-linear and dynamic, it is no surprise that non-replication left the field with few tenable brain phenotypes in ASD and even less insight into pathogenesis.
An increased understanding of the heritable nature of the disorder and recurrence risk in families6, led to a paradigm shift with the advent of the infant-sibling study design. Researchers began to follow the younger, high-risk siblings of older children with ASD – 20% of whom develop ASD themselves7 – through infancy and into toddlerhood, providing a window into the period when ASD first emerges8,9. These prospective studies have revealed that the diagnostic symptoms of ASD emerge during the latter part of the first and second year of life10–14. Differences in other developmental domains that are not necessarily specific to ASD, however, are detectable in the first year of life, including motor skills15–17, attention to faces and social scenes18–20, response to name21, visual reception15 and visual orienting22. Early in the second year of life, differences in language skills,9,15,23, and disengagement of visual attention24 are also evident.
These behaviors arise during a highly dynamic period of postnatal brain growth25,26, marked by cortical expansion27,28, fiber myelination and maturation29,30, and functional organization of neural circuitry31,32. Infant-sibling studies incorporating neuroimaging at the large-scale have provided great insight into brain development in ASD, revealing that atypical brain phenotypes emerge during infancy, with altered developmental trajectories preceding the consolidation of symptoms that begins in the second year of life33. This body of work has enhanced our understanding of the developmental time course of early ASD, and recently demonstrated the possibility of using presymptomatic magnetic resonance imaging (MRI) in infants to predict diagnostic outcomes in toddlerhood34,35, an advancement with important implications for clinical practice.
In this article, we review neuroimaging studies of early ASD including structural, diffusion, and functional MRI from the early postnatal period through preschool. The goal of this review is to synthesize information across studies to identify biomarkers endorsed across samples, outline the developmental time course of the emergence of ASD-related neural phenotypes, and identify candidate biological mechanisms. Additionally, we outline recent studies using neural phenotypes and machine learning approaches to predict subsequent diagnosis and discuss the implications for clinical practice. This review will conclude with future directions for the field, including the need to identify individual-specific areas of developmental concern, parse etiologic heterogeneity using neurological features, incorporate indices of genetic variation into neuroimaging studies of early brain development, chart the co-occurrence of developmental brain and behavioral phenotypes in individuals, and continue to bridge in-vivo MRI with basic science to reveal mechanistic insights into the pathophysiology of ASD.
STRUCTURAL MRI
Brain Overgrowth
Brain overgrowth in ASD has been widely documented, dating to the first reports of the phenomenon using MRI in adolescents and adults with ASD over two decades ago36–38. These findings were later extended to young children39–47, with convergent evidence across studies suggesting that brain overgrowth was present by 2 years of age in children with ASD. Indirect evidence from head circumference measurements at birth and MRI in infancy and toddlerhood suggested that brain overgrowth was not present at birth, but emerged in the later part of the first year of life42. This finding was later confirmed using MRI in a cohort of 55 infants longitudinally examined from 6 to 24 months of age48, such that infants who developed ASD (n = 10) demonstrated faster rates of total brain volume growth resulting in increased brain volumes by 12 to 24 months of age compared to infants who did not develop ASD. A more recent, large-scale study (106 high-risk infants, 42 controls) has provided additional evidence for brain volume overgrowth between 12 and 24 months, and linked the rate of change in total brain volume during the second year of life to the severity of ASD-related social deficits34. Importantly, the authors decomposed cortical volume into cortical thickness and surface area to reveal that faster rates of cortical surface area growth from 6 to 12 months of age precedes brain overgrowth in the second year of life in infants who later developed ASD (n = 15)34. The rate of surface area expansion from 6 to 12 months was also correlated with total brain volume at 24 months of age. These findings directly support the hypothesis generated from prior work that cortical hyper-expansion drives brain overgrowth in ASD45. A machine learning approach to diagnostic classification using MRI measures at 6 and 12 months was also employed in this study34, and is discussed in detail below.
Cortical Surface Area, Thickness, and Gyrification
The surface area and thickness of the cortex have been differentially examined – as opposed to jointly examined in studies of cortical volume – in only a handful of studies of young children with ASD. In the first study of its kind, Hazlett and colleagues reported increases in the surface area of the frontal, temporal, and parietal lobes in 2-year-olds with ASD45, findings which were replicated in a sample of 3-year-old boys with ASD49. A more recent study demonstrated both accelerated rates of total cortical surface area expansion, and regionalized expansion in areas in the occipital, temporal, and frontal lobes in infants who later went on to develop ASD, with robust rates of expansion notable in the visual cortex34. Taken together, these findings support the pathological hyper-expansion of cortical surface area in ASD, with Hazlett and colleagues34 tracing its origins to the first year of life. All three studies34,45,49 found no evidence of differences in cortical thickness between infants and toddlers with ASD and controls. One study in 2- to 5-year-old boys stands in contrast, reporting no differences in surface area but increased thickness in some localized cortical areas50; this may be due to the relatively small sample size (66 ASD, 29 controls) given a wider developmental age-range, use of vertex-based image analysis pipelines (not employed by the other three studies), or lack of detection of a brain overgrowth phenotype in their sample. Cortical thickness differences have been observed in adolescents and adults with ASD, though the direction of effect varies51–53. By employing a mixed cross-sectional and longitudinal design including individuals with ASD and controls (ages 3 to 39 years), Zielinski and colleagues provided some clarity to these incongruent findings54. The authors reported greater cortical thickness across multiple brain regions in childhood, followed by a crossing of trajectories in middle childhood and finally reduced regional cortical thickness in early adulthood in individuals with ASD54,55. In light of reports in infants and toddlers, it is likely that aberrant patterns of cortical thickness in ASD emerge sometime after age 3 and follow a dynamic developmental pattern thereafter. Cortical gyrification patterns – which may reflect surface area expansion – in young children with ASD are largely unknown. One recent study in boys (105 with ASD, 49 controls) ages 3 to 5 years found that at age 3, boys with ASD had reduced gyrification in the fusiform gyrus56. A longitudinal examination revealed that local indices of gyrification in boys with ASD increased across the preschool period in regions in the temporal, frontal, and parietal lobes56, whereas local gyrification was generally stable or decreasing in typically-developing controls. This is consistent with other studies reporting increased gyrification in older children and adults with ASD57–61. Further studies in young children and infants will be needed to discern developmental gyrification patterns in early ASD.
Subcortical Structures
There has been considerable interest in the role of the amygdala, as a core region in the social brain, in the pathophysiology of ASD62, yet there have be relatively few studies exploring the development of the amygdala and other subcortical brain regions in early ASD. Sparks and colleagues40 found evidence of bilateral enlargement of the amygdala and hippocampus using MRI in a sample of 3 to 4-year-olds with ASD, though after adjusting for total brain volume, only amygdala volumes in a subset of children with more severe ASD remained significantly enlarged. A longitudinal follow-up of this cohort revealed that greater volumes in the right amygdala in toddlerhood related to poorer social and communication outcomes at age 663. Similar findings were reported in another study of toddlers with ASD64, where increased amygdala size correlated with the severity of social and communication deficits, with a particularly robust amygdala phenotype reported in girls with ASD. In a longitudinal investigation of brain-behavior associations in toddlers with ASD (ages 2 to 4 years), Mosconi and colleagues65 reported that amygdala enlargement was present and stable across the preschool period, but, in contrast to other earlier studies, found that increased amygdala volume conferred better joint attention among children with ASD. In a study of boys ages 18 to 42 months, several subcortical structures were found to have increased volume compared to typically-developing controls including the amygdala (20% larger), caudate nucleus, globus pallidus, and putamen66. More recently, Qui and colleagues67 reported bilateral caudate enlargement from 2 to 4 years of age compared to children with developmental delay, and Pote and colleagues reported an overall enlargement of subcortical regions in 4- to 6-month-old infants at high familial risk for ASD (including infants who did and did not develop ASD, n = 26 total, n = 4 with ASD), with greater volumes associated with increased restricted and repetitive behaviors at 36 months68. A study of infants at elevated familial risk for ASD found differential associations between amygdala, thalamus, and caudate volumes at age 1 and language abilities at age 2 in infants who were later diagnosed with ASD versus those with language delay only69, the authors suggest this is reflective of distinct neural mechanisms, and likely genetic and environmental risk factors, governing language development in infants with ASD.
Cerebellum
Cerebellar structural abnormalities measured by MRI are frequently reported in older children and adults with ASD70,71, though the direction of effect varies71. Similar inconsistencies have been reported in studies of infants and toddlers. A study of 3 to 4-year-olds found that children with ASD fell between typically developing children (lowest cerebellar volumes) and children with developmental delays (greatest cerebellar volumes)72; the authors explored associations between cerebellar volumes and child behavior and found no associations. Larger white matter volumes within the cerebellum in young children with ASD have also been reported41,43, as well as increased gray matter, though only in young females43. Several other studies, however, found no differences in cerebellar volumes between cases and controls (ages 18 months to 5 years) after adjusting for total brain size40,42,45. Taken together, these finding suggest that cerebellar abnormalities may exist, but future work will be needed to arrive at a consensus in the literature. Additionally, findings are highly dependent on statistical modeling, and studies should carefully control for overall brain size to ensure that findings of volumetric enlargement are specific to the cerebellum.
Corpus Callosum Morphology
The corpus callosum in older children, adolescents, and adults with ASD has been shown to be smaller in size when compared to controls73–75. Studies in young children ages 3 and up have found results consistent with these findings. A study in 3 to 4-year-olds found that midsagittal corpus callosum area was disproportionately small relative to total brain size in children with ASD compared to typically-developing controls, with reduced area throughout the structure76. A more recent longitudinal study of 3 to 5-year-olds echoed these findings, reporting that children with ASD had smaller regions dedicated to fibers projecting to the superior frontal cortex compared to typically-developing children77. In the only prospective study in infants, Wolff and colleagues78 found that corpus callosum area and thickness were significantly greater at 6 and 12 months, but not 24 months, in infants with familial risk who went on to develop ASD, with the most prominent group differences found in the anterior region of the corpus callosum connecting the prefrontal cortex. This study also found that cross-sectional measures of area and thickness at 6 months of age were correlated with degree of restricted and repetitive behaviors at 24 months in infants who developed ASD. Taken together, these finding suggest that the development of the corpus callosum reflects a dynamic process whereby the size of the corpus callosum in individuals who develop ASD is increased compared to controls in the first year of life, normalizes by age 2, and becomes smaller sometime in the third year of life.
Increased Extra-Axial Cerebral Spinal Fluid Volume
Recent studies have detected increased volumes of extra-axial fluid – defined as the cerebrospinal fluid occupying the subarachnoid space surrounding the cortical surface of the brain – in the first year of life in infants who go on to develop ASD. In the original study to describe this phenomenon in early postnatal life, Shen and colleagues48 prospectively assessed brain and behavioral development in a sample of 55 infants (33 at familial risk for ASD, 22 controls), reporting increases in extra-axial fluid volumes at 6 months which persisted through 24 months in infants who went on to develop ASD (n = 10). The authors also reported that extra-axial fluid volumes at 6 months were related to ASD severity at the diagnostic visit. These findings were replicated in a much larger independent cohort of infants (N = 343, 221 at familial risk for ASD, 122 controls)79, where those who went on to develop ASD (n = 47) had 18% more extra-axial fluid at 6 months when compared to controls. The authors also reported that extra-axial fluid was disproportionately increased (25% greater than controls) in infants who went on to have the most severe ASD symptoms79. Shen and colleagues80 extended these findings to a community-ascertained sample of 2 to 4-year-olds with ASD, reporting that increases in extra-axial fluid were nearly identical in children with ASD and familial risk and in children with ASD without familial risk, and persisted through age 3. The authors also found that increased extra-axial fluid was associated with greater sleep problems and lower non-verbal ability in children with ASD. Taken together, these three studies provide evidence that extra-axial fluid is a robust brain biomarker of ASD in early life that deserves further mechanistic study.
DIFFUSION MRI
White Matter Integrity and Connectivity
Using diffusion MRI, scientists have investigated white matter connectivity and integrity in ASD, though few studies have focused on the preschool period. In a small study of seven children ages 1 to 3 years, Ben Bashat and colleagues81 found higher fractional anisotropy (FA; reflects the degree of directed water diffusion in the brain, indicative of more mature white matter properties, including myelination, axonal density, and fiber packaging82) in corpus callosum, corticospinal tract, and internal and external capsule when compared to typically-developing children. These early findings were in contrast to studies in adults that generally reported reduced FA in individuals with ASD83, but later supported by additional independent studies. Weinstein and colleagues84 reported that young children under the age of six with ASD had increased FA in many fiber tracts compared to controls, including the cingulum, corpus callosum, and superior longitudinal fasciculus. Xiao and colleagues47 reported similar findings, with increased FA in the corpus callosum, cingulum, and limbic system in toddlers with ASD. Another study reported increased FA in the frontal, temporal, and subcortical regions in young children with ASD (n = 32) compared to those with developmental delay (n = 16)85. The authors also reported an over-connectivity phenotype in ASD, though the methodology employed uses direct streamline counts as a measure of connectivity strength, which has limitations86,87. Another more recent study of 97 toddlers (68 with ASD, 29 controls), found that FA in the corpus callosum fibers projecting to the temporal lobes was significantly greater in toddlers with ASD88. Two other studies found opposite patterns of FA in preschoolers with ASD versus controls. One cross-sectional study in 2 to 6-year-olds with ASD reported reduced FA in children with ASD compared to controls (including both typically developing children and children with developmental delay)89, which is in contrast with other cross-sectional studies, possibly due to collapsing across a relatively wide age range. Another found lower FA in toddlers and children with ASD (mean age 5 years, ranging from 2 to 11 years), again possibly due to collapsing across a wide developmental range90.
Two longitudinal studies have provided clarity, revealing the dynamic developmental nature of white matter development in ASD. Wolff and colleagues91 utilized an infant-sibling research design to prospectively follow 92 infants at familial risk for ASD at 6, 12, and 24 months of age. The authors reported widespread significant differences in growth trajectories in major white matter fiber bundles in infants who went on to develop ASD (n = 28) compared to those who did not. Infants later diagnosed with ASD exhibited increased FA at 6 months of age followed by slower maturation through 24 months of age. In line with the study by Wolff and colleagues91, a study of 1 to 4-year-olds reported abnormal age-related changes in FA, with greater FA at younger ages and slower rates of change thereafter, especially in frontal fiber tracts92. Taken together, these findings suggest that ASD is characterized by increased FA in the first year of life, marked be a slowing in maturation thereafter that may ultimately result in reduced FA values observed in older children and adults.
Newer methodological approaches have been used to consider white matter in the human brain as a network, or connectome93. Lewis and colleagues94 estimated properties of white matter network efficiency in 2-year-olds and found that toddlers with ASD had reduced local and global efficiency, especially in sensory processing regions in the occipital and temporal lobes, compared to controls. In a follow-up study the authors downward extended these findings to reveal deficits in white matter network efficiency as early as 6 months of age in infants who went on to develop ASD95.
There is a growing body of work linking white matter development and ASD-related behaviors in young children with ASD. Wolff and colleagues96 recently reported that developmental changes in FA in cerebellar fibers and the corpus callosum in infants with ASD was positively associated with restricted and repetitive behaviors and response to sensory stimuli. Another study found that visual orienting at 7 months of age was associated with the microstructural organization of the splenium, but only in children without an ASD diagnosis, possibly suggesting an aberrant functional specialization of visual circuitry in ASD97. Lewis and colleagues demonstrated the inefficiencies in white matter connectivity, especially in longitudinally in temporal regions, was associated with symptom severity at 24 months95, findings echoed in a study by Fingher and colleagues88 reporting that white matter integrity in the temporal segments of the corpus callosum in toddlers was associated with outcome measures of ASD severity at later ages. White matter correlates of language were studied in 104 preschool-aged boys with ASD98, and authors reported that FA (and other measures of microstructure) in the bilateral inferior longitudinal fasciculus both differed within the ASD group based on level of language, and was associated with individual differences in language scores. In another recent study of language and white matter development, Liu and colleagues99 reported altered lateralization patterns in language tracts in infants at familial risk for ASD (n = 16), with FA lateralization in 6-week-olds relating to language outcomes at 18 months and ASD symptomology at 36 months, though it is unclear how this relates to symptomology above the diagnostic threshold. These findings suggest that behavioral disruptions in ASD may result from a variety of alterations in white matter development that deserve further investigation.
FUNCTIONAL MRI
Auditory-Evoked fMRI
Functional MRI (fMRI) studies assessing spontaneous fluctuations in blood oxygenation as an index of neural activity and connectivity in very young children with ASD were relatively sparse until recently. The first auditory-evoked fMRI study during natural sleep in young children with ASD found significant differences in brain activation in a distributed network in response to forward and backward speech stimuli100, with a rightward lateralization in speech perception networks in toddlers with ASD (n = 12). Using the same experimental approach, Eyler and colleagues101 found that neural response to sound in infants ages 12 to 48 months later diagnosed with ASD (n = 40) was deficient in the left hemisphere and, again, abnormally right-lateralized in the temporal lobe compared to typically-developing controls. In a follow-up study, Dinstein and colleagues102 investigated spontaneous activity (by regressing out stimulus structure) to find that toddlers with ASD had significantly weaker interhemispheric synchronization in putative language areas including the inferior frontal gyrus. The strength of synchronization in the inferior frontal gyrus was positively correlated with verbal ability and negatively correlated with ASD severity. A more recent study by this group examining activation patterns found that toddlers with ASD who had poorer language performance a year later exhibited reduced activation in bilateral temporal and frontal brain regions when compared to controls103. Further, the authors reported inverse and differential brain-behavior associations between the ASD groups and controls, suggesting aberrant functional specialization of language regions in ASD, in line with previous work.
Resting State fMRI
In a study of whole-brain resting state functional connectivity, Chen and colleagues104 revealed two atypical circuits in young children with ASD (n = 58, 29 with ASD, mean age 4.98 years, all children sedated): one comprised of brain regions involved in social cognition exhibiting under-connectivity and the other comprised of sensory-motor and visual brain regions showing over-connectivity in ASD. The authors employed support vector regression analysis to show that the two circuits were differentially related to, and predictive of, individual social deficits and restricted behaviors in their sample. Another study investigating social brain network function found that newborns with a family history of ASD (n = 18) exhibited significantly greater neural activity in the right fusiform and left parietal cortex, and altered age-related changes in activity in the cingulate and insula105, though it is unclear whether these patterns are specific to the development of early ASD, as the diagnostic outcome of the infants was not reported. Weakened functional connectivity of the amygdala and several brain regions involved in social communication and repetitive behaviors has also been reported in young boys with ASD (mean age 3.5 years)106. Repetitive behaviors and whole-brain functional connectivity was recently investigated in a study of infants at familial risk for ASD (n = 38)107. The authors found age-specific associations between functional connectivity in visual, control, and default mode networks, such that weaker positive correlations in activity at 12 months and between 12 and 24 months were associated with more restrictive and repetitive behaviors in infants at risk for ASD (n = 38, 20 went on to develop ASD). The direction of this association was reversed at age 2, such that more positive correlations between dorsal attention, subcortical, and default mode networks were associated with more restrictive and repetitive behaviors. The promise of resting-state connectivity as an ASD biomarker was recently demonstrated, where functional connectivity features in 6-month-olds (derived from connections associated with ASD-relevant behaviors) were able to accurately predict diagnostic outcome at 24 months of age35. This study is described in detail below.
NEUROIMAGING FINDINGS IN A DEVELOPMENTAL CONTEXT
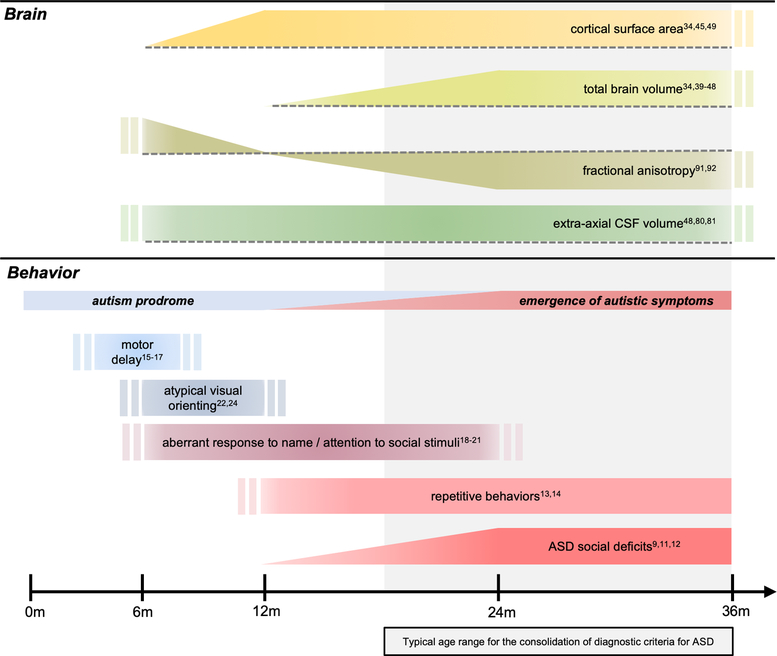
With growing evidence of brain changes in ASD preceding the emergence of the defining features of the disorder, it becomes critical to place these prodromal brain phenotypes in the context of early-emerging behaviors associated with ASD and ASD risk. Here we have developed a schematic (Figure 1) demonstrating key findings from the neuroimaging literature reviewed above placed in a developmental context alongside behavioral and clinical phenotypes. Aberrant white matter development (indicated by fractional anisotropy108 and corpus callosum size109) and increased extra-axial cerebrospinal fluid (CSF) volumes48,79,110 are detectable by 6 months of age in infants who go on to develop ASD. This coincides with motor delays15–17, atypical visual orienting97, and aberrant attention to social stimuli18,20. It is important to note, however, that motor delays do not appear to be specific to children with ASD, and are also evident in high-risk infant siblings who do not meet diagnostic criteria16,111.
Figure 1. Summary of neuroimaging findings in ASD in the context of emerging behaviors from infancy through toddlerhood.
Brain changes in ASD precede the development of the defining diagnostic features of the disorder, and are temporally associated with behavioral changes in the first year of life that are both specific and non-specific to ASD. Aberrant white matter integrity (fractional anisotropy) and increased extra-axial cerebrospinal fluid (CSF) volumes are detectable as early as 6 months of age in infants who go on to develop ASD, concurrent with motor and sensory delays. Surface area hyper-expansion in the first year of life precedes brain overgrowth in the second year, during which time ASD symptoms become apparent and begin to consolidate, while brain phenotypes remain relatively stable. Findings presented in the figure are those which are supported by multiple study paradigms (reference numbers noted in the figure), including at least one longitudinal study per phenotype. Double bars indicate that the start and/or end point of the trajectory is unknown or not well documented in the literature. Dashed lines in the top panel represent a reference to typical brain development, where bars above or below the dotted line indicate the brain phenotype is either increased or decreased relative to controls, respectively. For example, fractional anisotropy in ASD is increased at 6 months, not significantly different at 12 months, and decreased from 24 to 36 months when compared to controls. Repetitive behaviors and ASD social deficits are shown to continue past 36 months without citations, as these are diagnostic features that are, by definition, present in individuals with an ASD diagnosis.
Surface area hyper-expansion in the first year of life precedes brain overgrowth in the second year34. Concurrently, infants who go on to develop ASD exhibit altered response to name beginning at 9 months and continuing through 24 months21, coinciding with differential trajectories in attention to eyes compared to controls19, and the emergence of ASD symptoms9,11–14. Taken together, these results begin to build a developmental timeline in which brain and behavioral phenotypes associated with ASD and ASD risk emerge during a prodromal period largely prior the second birthday, after which time diagnostic symptoms begin to consolidate.
CANDIDATE NEUROBIOLOGICAL MECHANISMS
The first two years of life are marked by rapid, dynamic brain growth, with total brain volume doubling in the first year112, largely driven by gray matter development, and specifically the expansion of cortical surface area27. In ASD, however, this postnatal developmental trajectory is disrupted. Findings from behavioral and neuroimaging studies of infants who go on to develop ASD suggest that the hyper-expansion of cortical surface area co-occurs with a prodromal period of motor, sensory, and visual orienting deficits observed from 6 to 12 months of age, followed by brain overgrowth and the emergence of autistic social deficits in the second year of life2. This highlights a central role for mechanisms governing surface area expansion in the pathophysiology of ASD.
The expansion of cortical surface area is thought to be governed by neural progenitor cell proliferation, differentiation, and migration113–116, with updated models specific to the gyrencephalic cortex pointing to the role of the fan-like expansion of outer radial glial (oRG) cells in tangential surface area growth114,115. The expansion of the oRG cell population is directly related to brain size113, as oRG give rise to highly proliferative intermediate progenitor cells that undergo amplifying divisions during neurogenesis117. Evidence for the potential role of neural progenitor proliferation and neurogenesis in the development of ASD has been supported by a wealth of preclinical, genetic, and postmortem data reviewed in detail elsewhere118. It is further supported by recent studies demonstrating that neural progenitor cells derived from individuals with ASD display excess proliferation compared to controls119,120, with the level of proliferation relating to the degree of brain overgrowth observed using MRI119. Another similarly-designed study found evidence of significant developmental acceleration in neuronal differentiation in ASD, resulting in neurons with more complex branching121. Increased brain volume and macrocephaly are hallmarks of several genetically-defined autistic syndromes including 16p11 deletion, PTEN, and Chd8 mutations122–125, providing a window into the underlying pathophysiology in at least a subset of individuals with ASD.
There is evidence that the overproduction of neurons alters neural connectivity, with downstream consequences for circuit function and behavior. In mice, the induced overpopulation of upper-layer pyramidal neurons disrupts the development of dendrites and spines and alters the laminar distribution of neurons, resulting in dysregulated synaptic connectivity and autism-like behaviors126. This aligns with studies reporting alterations in synaptogenesis and neuronal excitability119, and relatively more inhibitory neurons and synapses in organoids derived from cells of ASD patients with macrocephaly120. Another preclinical study observed postnatal brain overgrowth, altered long-range functional connectivity, motor delay, and anomalous response to social stimuli in Chd8 mutant mice, suggesting that altered brain growth and disrupted long-range wiring may underlie behavioral deficits observed in at least some subtypes of ASD127. Other evidence suggests that brain overgrowth in ASD may also be related to alterations in mechanisms governing synaptic pruning and the refinement of neural circuitry that occurs during early postnatal development128. Experience-dependent plasticity has particularly notable impacts on primary sensory systems129 which also exhibit surface area hyper-expansion in infants who go on the develop ASD34. Deficits in the cellular mechanisms controlling experience-dependent elimination of synapses – specifically long-term depression (LTD) – has been observed in several mouse models of ASD and related neurodevelopmental disorders130–133. Further, locally-balanced excitation and inhibition play a key role in modulating competition between synapses and ultimately in defining the critical period for plasticity and refinement129; an imbalance in excitatory and inhibitory synapses like that reported by Marchetto and colleagues119 in ASD-derived neuronal cultures could have marked impacts on the development of neural circuitry.
When considering brain overgrowth and behavioral findings together, a picture emerges of how ASD may develop in early life. Cortical hyper-expansion from 6 to 12 months, especially in the visual cortex34, may underlie concurrent deficits in visual orienting behaviors19,134, in turn altering experience-dependent neuronal development and ultimately resulting in inefficiently pruned circuits, brain overgrowth, and the emergence of ASD traits2. Though, it is also possible that brain volume overgrowth is secondary to the increase in intermediate progenitor cells and less influenced by experience-dependent pruning mechanisms.
Neuroimaging findings of increased extra-axial fluid volumes implicate additional pathogenic mechanisms in ASD. A body of recent work has elucidated the role of cerebrospinal fluid (CSF) in brain development and function135. Lehtinen and colleagues136 found that CSF contained growth factors with age-dependent effects on neuronal proliferation, suggesting an important role for CSF composition in cortical development. Further, increased volumes of extra-axial fluid suggest a disruption in the circulation of CSF and an accumulation of brain metabolites that impact brain function including amyloid beta and pro-inflammatory cytokines137,138. These findings, coupled with evidence that extra-axial fluid volume is increased prior to surface area hyper-expansion48,79, implicates the functional role of CSF in pathophysiology of ASD and related neurodevelopmental disorders. Preclinical work will be needed to further explore a potential regulatory role for CSF in surface area hyper-expansion in ASD.
Alterations in corpus callosum morphology and in the development of white matter microstructure in early ASD implicates processes governing myelination, axon caliber, density and axonal connectivity. A study of several mouse models of ASD recently identified a significant enrichment of myelination genes, and gene-set analysis implicated genes and pathways associated with myelination and oligodendrocyte differentiation139. Altered oligodendrocyte function has been documented in Pten-mutant mouse models of ASD, such that oligodendrocyte progenitor cells developed too early, resulting in reduced myelin sheaths140, which would impede information transfer along axons. The finding of reduced myelin sheath thickness has also been observed in postmortem studies of individuals with ASD141. This same study141 also reported a decrease in large-diameter long-range axons and an increase in small-diameter short-range axons in the frontal cortex, consistent with inefficient connectivity observed in imaging studies in infants and toddlers with ASD94,95. White matter integrity and connectivity may also be altered through experience-dependent myelination142,143, where oligodendrocytes selectively myelinate axons which receive more input from neurons, in line with altered excitability observed in neurons derived from ASD patients119.
In summary, it is likely that ASD arises from multiple pre- and postnatal pathogenic mechanisms involving neural proliferation and migration, synaptogenesis, pruning, myelination, and axonal development and connectivity – with each of these processes having important independent and interactive contributions to brain development. This is no surprise, as a large-scale genetic study of over 18,000 individuals with ASD identified that one’s risk for ASD depends on the level of polygenic burden of thousands of common variants in a dose-dependent manner144. Further, many of the genes implicated in ASD are pleiotropic in nature, impacting numerous cellular and molecular pathways145. This, coupled with what is known about the development of ASD from neuroimaging studies, suggests an early-emerging vulnerability that is non-specific in nature with effects on brain development detectable as early as the first year of life. This mechanistic complexity likely underlies the notable behavioral and clinical variability observed in ASD, calling for a need to parse phenotypic heterogeneity in order to arrive at more parsimonious etiological models.
PREDICTING ASD DIAGNOSIS
Presymptomatic Prediction using MRI
Two recent studies employing a prospective longitudinal design coupled with machine learning approaches demonstrated the potential for predicting ASD diagnosis at 24 months using infant MRI scans collected in the first year of life. Both studies followed younger siblings of older children with ASD from 6 months of age and collected MRI scans, behavioral measures, and clinical outcomes. In the first study, the authors used supervised deep learning to build a classification algorithm that relied primarily on measures of regional cortical surface area growth from 6 to 12 months of age to predict ASD diagnostic outcome at 24 months34. This algorithm correctly predicted diagnosis in a sample of 106 infants at risk for ASD (15 received a diagnosis at 24 months) with 88% sensitivity, 95% specificity, and a positive predictive value (PPV) of 81%. This study is notable for two major reasons: (1) it significantly outperformed behavioral measures in the first two years in predicting diagnostic outcome146–149, and (2) it used features derived from a standard structural MRI preceding the onset of the defining behavioral features of the disorder, demonstrating the possibility of assigning infants to pre-symptomatic intervention during a period of heightened neural plasticity. The other study from the same group found that a support vector regression machine using whole-brain functional connectivity matrices – culled to connections significantly correlated with 24-month scores on measures of social behavior, language, motor development, and repetitive behavior – could predict diagnostic outcome with 82% sensitivity, 100% specificity, and a PPV of 100% in a sample of 59 high-risk infants, 11 of which received a diagnosis35. Both of these studies pave the way for larger-scale investigations of presymptomatic diagnostic classification using MRI.
Diagnostic Prediction with MRI: Best Practices in an Emerging Field
Machine learning, and particularly deep learning, have recently taken on a prominent role in neuroimaging research by allowing for the design of powerful classifiers able to exploit complex relationships between brain structural and functional features and cognitive and clinical phenotypes150. Several supervised discriminative machine learning methods have gained popularity for use with MRI datasets, perhaps the most popular (especially in the context of low-dimensional and limited datasets), being support vector machines (SVM), followed by, more recently, deep learning (DL). Following a feature reduction step, SVM works by finding the optimal linear plane separating classes (i.e., diagnostic groups) using the original data (linear SVM), or data mapped into a new feature space using pre-defined kernel functions (non-linear SVM) where classes become linearly separable151. On the other hand, with the increasing availability of larger neuroimaging datasets, DL algorithms have shown success in automatically identifying the optimal data representation in a data-driven manner, bypassing the need for prior selection of an appropriate non-linear mapping150. This distinction is evidenced in the two studies mentioned above, where Hazlett and colleagues34 used a DL approach that did not require a separate feature reduction step prior to, or separate from, building the algorithm, whereas Emerson and colleagues35 reduced their connectivity features to those correlated with behavior prior to building the SVM classifier. Potential advantages of DL methods over SVM include the fact that input features are learned from the data and not derived, which is less prone to overfitting, and the ability of DL to achieve a higher level of abstraction and complexity, allowing for the detection of more subtle patterns in the data150. For further information on machine learning algorithms used in pediatric neuroimaging, see a recent review by Mostapha and Styner152. Regardless of the approach taken, these methods should be employed with the oversight of an experienced artificial intelligence (AI) scientist, statistician, or an engineer who regularly applies machine learning algorithms to high-dimensional datasets. Equally importantly, insight from individuals with clinical knowledge of the disorder will be critical in interpreting the complex results generated from these types of models.
With regards to best practices for conducting neuroimaging-based prediction studies, several key topics emerge, including sample size and generalizability, interpretation, and methodological transparency. Sample size is a major factor in designing accurate, generalizable supervised classification algorithms, particularly when dealing with MRI datasets that are as heterogeneous as those observed in early postnatal development152. Future work using large, publicly available datasets – with compatible MRI sequences, age windows, and serial scans – will help combat this problem, alongside employing rigorous cross-validation methods to ensure that the trained models generalize to unseen data. Class-imbalance is another major issue in predicting outcomes with low prevalence in the population. Algorithms tend to optimally recognize classes (or outcomes) with larger training samples, as opposed to minority samples with fewer training samples152, as would be the case with predicting an ASD diagnosis. There are new methods on the rise for addressing these concerns, including synthetic oversampling strategies153,154. Once classification algorithms are built and tested, it is scientifically critical to understand which features derived from the MR images (i.e., which brain connections or regions) contributed to the classification. At the moment, it is still challenging to interpret what deep learning models have learned, although methods to solve this problem are increasingly proposed152, including backtrack methods like the one employed by Hazlett and colleagues34. Finally, in order to share knowledge and create standards for best practice in the field, transparency is needed in the reporting and sharing of machine learning algorithms used in publications. Authors should outline the rationale for the selection of the machine learning algorithm employed in the study and report sample sizes, cross-validation and training, and testing procedures. Steps taken to address class-imbalance and details regarding tuning and optimization parameters should also be noted. Finally, the steps taken to interpret the findings, including methods used for identifying information learned by the algorithm and clinically-relevant performance metrics (specificity, sensitivity, positive predictive value) should be included. The code used for building algorithms and conducting analyses must be made readily available to others for verification and replication.
Clinical and Ethical Considerations
Presymptomatic, individualized prediction at the large scale has substantial implications for shaping clinical practice, yet it comes with ethical implications155 that must be carefully considered. The transition from group-level correlations to individual-level prediction in neuroscience is a key step towards improving the lives of individuals, and begins with carefully replicating pioneering studies by applying their models to new, independent datasets156. The development of psychoradiology, however, has shown promise in this regard aiming to achieve the individualized prediction for psychiatric disorders157–161, The next step is to integrate validated algorithms into clinical practice, in keeping with the precision medicine framework designed to assign individuals to personal treatment plans, maximizing treatment efficacy162. While there are some evidence-based behavioral interventions for early ASD163–166, pre-emptive intervention has yet to be proven successful167. This both highlights the urgent need for developing and testing presymptomatic interventions, and raises the concern of implementing early diagnostic screening if no validated treatment options are available. Neuroimaging should be harnessed as a biologically-based screening tool that may offer insights into when and how to intervene, guiding future research.
FUTURE DIRECTIONS
Predicting Dimensional Outcomes
A major next step for the field will be to develop methodologies to predict individualized areas of concern, as ASD and other neurodevelopmental disorders exhibit substantial phenotypic variability. Additionally, more than one quarter of infants at familial risk for ASD will develop subthreshold atypical behaviors in the first years of life111, and could also be candidates for targeted intervention. Neuroimaging studies employing machine learning approaches have demonstrated the possibility of individualized prediction of cognitive outcomes in toddlerhood using neonatal diffusion MRIs168,169; future work should consider applying similar methodologies to infants at risk for ASD. Using MRI to target intervention to the first year of life may be most beneficial, as behaviors appear to be more separable and potentially more targetable in infancy170.
Parsing Heterogeneity
ASD has a strong heritable component, but complex genetic origins that overlap with other neuropsychiatric disorders, calling for a need to move beyond the traditional clinical diagnostic model to one increasingly guided by biology171. However, while heterogeneity in brain functioning is observable in psychiatric disorders and across individuals172–175, it is rarely considered in experimental designs. Parsing heterogeneity in neurodevelopmental profiles is likely a promising avenue for improving our understanding of the diversity and variability in symptomology associated with complex neuropsychiatric disorders, and is a major focus of the NIMH Research Diagnostic Criteria (RDoC) project176. Novel approaches to implementing clustering algorithms to identify subgroups in the population based on neural features has great promise to reveal meaningful insight into both etiology and treatment. In a similar manner, a developmental approach should be taken to identify subgroups with similar trajectories of the disorder, likely to be reflective of distinct etiologies177.
Relating ASD Genetic Liability to Neurodevelopment
While significant advances in genetics have identified de novo mutations in a portion of the ASD population, common178,179, additive178,180 polygenic variation is thought to account for the vast majority of ASD cases. It is currently unknown how heritable common background genetic variation and polygenic risk for ASD contribute to individual differences in brain development during infancy and toddlerhood. The familial nature of the infant-sibling study design is well suited to explore these associations. Recent work in syndromic ASD has demonstrated the predictive power of background genetic for behavioral development in young children181, and future studies should extend this to idiopathic ASD, using neuroimaging to reveal etiological insights into the early behavioral manifestation of the disorder.
Identifying Developmental Associations between Brain and Behavior Phenotypes
Infants who go on to develop ASD – as a group – exhibit a variety of brain phenotypes, including brain overgrowth, increased extra-axial fluid volumes, abnormal development of the corpus callosum and other white matter pathways, and altered functional brain connectivity patterns. None of these phenotypes, on their own, are sufficient to predict diagnosis or identify causal mechanisms, pointing to multiple etiologies both within and between individuals. To date, we do not have a clear understanding of how these brain phenotypes are related in individuals, or how they link to behavior. Some of the earliest behaviors disrupted in infants who go on to develop ASD include motor skills, which have notable implications for later-emerging language and communication abilities182–184. Charting the developmental co-emergence and co-occurrence of brain and behavioral phenotypes in ASD from infancy through diagnosis should be a major scientific goal in the next generation of infant-sibling studies. Such detailed phenotypic developmental mapping would greatly improve our understanding of the unfolding of ASD, possibly revealing distinct etiological subgroups.
Linking MRI and Basic Science
Recently, substantial strides in basic science have allowed for the use of neural stem cells to recapitulate in-vivo brain development in-vitro. Several reports reviewed here used cells derived from individuals with ASD and macrocephaly to mimic early prenatal cortical development119–121. These studies represent an important step for the field in relating brain phenotypes observed in MRI to in-vitro models derived from the same individuals, though methodological advances will be needed to allow for modeling later stages of brain development185 that may be more central to ASD33. Future work should move beyond only studying individuals with ASD and brain overgrowth phenotypes119–121 to reveal broader insights into etiology.
SUMMARY
Brain phenotypes derived from neuroimaging provide the earliest distinction between infants at risk for ASD and typically developing children, with group differences noted during the presymptomatic period before aberrant behavior is reliably detectable. A wealth of studies converge upon several key findings including brain overgrowth, increased extra-axial fluid volumes, altered white matter development, and aberrant structural and functional connectivity patterns in individuals with ASD. This implicates a variety of neurobiological mechanisms that both independently and jointly contribute to brain and behavioral development in early childhood. The field has made significant strides in describing brain phenotypes in ASD, and has recently taken steps towards implementing individualized-prediction models to identify infants at heightened risk for developing ASD, calling for an urgent need for the concurrent development of effective presymptomatic interventions. In the coming years, scientists will need to focus on a variety of key areas for further investigation, including tackling the problem of etiological heterogeneity and linking brain and behavioral development to underlying genetic mechanisms, a goal that will be achieved through a multidisciplinary approach combining neuroimaging, behavioral, and basic science research.
KEY POINTS.
Neuroimaging has played a key role in revealing brain phenotypes associated with autism spectrum disorder (ASD) during infancy and toddlerhood.
A wealth of studies converge upon several key findings including brain overgrowth, increased extra-axial cerebrospinal fluid volume, altered white matter development, and aberrant structural and functional connectivity patterns in ASD.
It is likely that ASD arises from multiple pre- and postnatal pathogenic mechanisms involving neural proliferation and migration, synaptogenesis, pruning, myelination, and axonal development and connectivity.
Predicting diagnostic and dimensional outcomes using neuroimaging data in infancy holds great promise for advancing clinical practice.
Future work should focus on parsing heterogeneity in ASD, linking genetic variation to brain imaging data in infancy, charting the co-occurrence of developmental brain and behavior phenotypes, and coupling neuroimaging studies with basic science research.
ACKNOWLEDGEMENTS
We thank Mahmoud Mostapha and Martin Styner for their scientific overview as experts in applying deep learning methods to infant neuroimaging datasets.
DISCLOSURE STATEMENT
Jessica B. Girault is funded by NIH T32-HD040127 to JP. JP is funded by NIH R01-HD055741, R01-MH118362, and U54-HD079124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Baio J, Wiggins L, Christensen DL, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry 2017;22(10):1385–1394. doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff JJ, Jacob S, Elison JT. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Dev Psychopathol 2018;30(2):479–495. doi: 10.1017/S0954579417000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff JJ, Piven J. On the emergence of autism: neuroimaging findings from birth to preschool. Neuropsychiatry 2013;3(2):209–222. doi: 10.2217/npy.13.11. [DOI] [Google Scholar]
- 5.Swanson MR, Piven J. Neurodevelopment of autism: the first three years of life In: Casanova MF, El-Baz A, Suri JS, eds. Autism Imaging and Devices Boca Raton, FL: CRC Press; 2017. [Google Scholar]
- 6.Szatmari P, Jones MB, Zwaigenbaum L, MacLean JE. Genetics of autism: overview and new directions. J Autism Dev Disord 1998;28(5):351–368. [DOI] [PubMed] [Google Scholar]
- 7.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 9.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozonoff S, Iosif A-M, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 2010;49(3):256–66.e1–2. [PMC free article] [PubMed] [Google Scholar]
- 12.Landa RJ, Gross AL, Stuart EA, Faherty A. Developmental Trajectories in Children With and Without Autism Spectrum Disorders: The First 3 Years. Child Dev 2012;84(2):429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elison JT, Wolff JJ, Reznick JS, et al. Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2014;53(11):1216–1224. doi: 10.1016/j.jaac.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff JJ, Botteron KN, Dager SR, et al. Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry 2014;55(8):945–953. doi: 10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes A, Zwaigenbaum L, Gu H, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord 2015;7(1):24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iverson JM, Shic F, Wall CA, et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J Abnorm Psychol 2019;128(1):69–80. doi: 10.1037/abn0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: a preliminary study. Am J Occup Ther 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- 18.Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol Psychiatry 2013;74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biol Psychiatry 2014;75(3):231–237. doi: 10.1016/j.biopsych.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller M, Iosif A-M, Hill M, Young GS, Schwichtenberg AJ, Ozonoff S. Response to Name in Infants Developing Autism Spectrum Disorder: A Prospective Study. J Pediatr 2017;183:141–146.e141. doi: 10.1016/j.jpeds.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elison JT, Paterson SJ, Wolff JJ, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson MR, Shen MD, Wolff JJ, et al. Subcortical Brain and Behavior Phenotypes Differentiate Infants With Autism Versus Language Delay. Biol Psychiatry Cogn Neurosci Neuroimaging 2017;2(8):664–672. doi: 10.1016/j.bpsc.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH. Disengagement of Visual Attention in Infancy is Associated with Emerging Autism in Toddlerhood. Biol Psychiatry 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience 2018;19(3):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullins J, Jha SC, Knickmeyer RC, Gilmore JH. Brain Development during the preschool period In: Joan Luby, ed. Handbook of Preschool Mental Health Guilford Press Press; 2016. [Google Scholar]
- 27.Lyall AE, Shi F, Geng X, et al. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb Cortex 2015;25(8):2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Wang L, Shi F, et al. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J Neurosci 2014;34(12):4228–4238. doi: 10.1523/JNEUROSCI.3976-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girault JB, Cornea E, Goldman BD, Knickmeyer RC, Styner M, Gilmore JH. White matter microstructural development and cognitive ability in the first 2 years of life. Hum Brain Mapp 2018;111(20):7456. doi: 10.1002/hbm.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng X, Gouttard S, Sharma A, et al. Quantitative tract-based white matter development from birth to age 2 years. Neuroimage 2012;61(3):542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. Development of human brain cortical network architecture during infancy. Brain Structure and Function 2015;220(2):1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao W, Lin W, Grewen K, Gilmore JH. Functional Connectivity of the Infant Human Brain: Plastic and Modifiable. The Neuroscientist February 2016. doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry 2017;22(10):1385–1394. doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazlett HC, Gu H, Munsell BC, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emerson RW, Adams C, Nishino T, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med 2017;9(393):eaag2882. doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. AJP 1995;152(8):1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- 37.Piven J ARNDT S, BAILEY J, ANDREASEN N Regional Brain Enlargement in Autism: A Magnetic Resonance Imaging Study. J Am Acad Child Adolesc Psychiatry 1996;35(4):530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biol Psychiatry 1992;31(5):491–504. [DOI] [PubMed] [Google Scholar]
- 39.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 40.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 41.Akshoomoff N, Lord C, Lincoln AJ, et al. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry 2004;43(3):349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 43.Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry 2007;46(4):515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- 44.Nordahl CW, Lange N, Li DD, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci USA 2011;108(50):20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazlett HC, Poe MD, Gerig G, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumann CM, Bloss CS, Barnes CC, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao Z, Qiu T, Ke X, et al. Autism spectrum disorder as early neurodevelopmental disorder: evidence from the brain imaging abnormalities in 2–3 years old toddlers. J Autism Dev Disord 2014;44(7):1633–1640. doi: 10.1007/s10803-014-2033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen MD, Nordahl CW, Young GS, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 2013;136(Pt 9):2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohta H, Nordahl CW, Iosif A-M, Lee A, Rogers S, Amaral DG. Increased Surface Area, but not Cortical Thickness, in a Subset of Young Boys With Autism Spectrum Disorder. Autism Research 2016;9(2):232–248. doi: 10.1002/aur.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raznahan A, Lenroot R, Thurm A, et al. Mapping cortical anatomy in preschool aged children with autism using surface-based morphometry. NeuroImage: Clinical 2013;2:111–119. doi: 10.1016/j.nicl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. AJP 2006;163(7):1290–1292. doi: 10.1176/ajp.2006.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp 2009;10(6, Part 1):NA–NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex 2006;16(9):1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 54.Zielinski BA, Prigge MBD, Nielsen JA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 2014;137(Pt 6):1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolff JJ, Piven J. Neurodevelopmental disorders: Accelerating progress in autism through developmental research. Nat Rev Neurol 2014;10(8):431–432. doi: 10.1038/nrneurol.2014.126. [DOI] [PubMed] [Google Scholar]
- 56.Libero LE, Schaer M, Li DD, Amaral DG, Nordahl CW. A Longitudinal Study of Local Gyrification Index in Young Boys With Autism Spectrum Disorder. Cereb Cortex 2018;33(6):15004–2587. doi: 10.1093/cercor/bhy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams EL, El-Baz A, Nitzken M, Switala AE, Casanova MF. Spherical harmonic analysis of cortical complexity in autism and dyslexia. Transl Neurosci 2012;3(1):36–40. doi: 10.2478/s13380-012-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohli JS, Kinnear MK, Fong CH, Fishman I, Carper RA, Müller R-A. Local Cortical Gyrification is Increased in Children With Autism Spectrum Disorders, but Decreases Rapidly in Adolescents. Cereb Cortex 2019;29(6):2412–2423. doi: 10.1093/cercor/bhy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res 2004;131(3):263–268. doi: 10.1016/j.pscychresns.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Nordahl CW, Dierker D, Mostafavi I, et al. Cortical Folding Abnormalities in Autism Revealed by Surface-Based Morphometry. Journal of Neuroscience 2007;27(43):11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shokouhi M, Williams JHG, Waiter GD, Condon B. Changes in the sulcal size associated with autism spectrum disorder revealed by sulcal morphometry. Autism Research 2012;5(4):245–252. doi: 10.1002/aur.1232. [DOI] [PubMed] [Google Scholar]
- 62.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience & Biobehavioral Reviews 2000;24(3):355–364. [DOI] [PubMed] [Google Scholar]
- 63.Munson J, Dawson G, Abbott R, et al. Amygdalar Volume and Behavioral Development in Autism. Arch Gen Psychiatry 2006;63(6):686. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- 64.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry 2009;66(10):942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry 2009;66(5):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hazlett HC, Poe MD, Lightbody AA, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord 2009;1(1):81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu T, Chang C, Li Y, et al. Two years changes in the development of caudate nucleus are involved in restricted repetitive behaviors in 2–5-year-old children with autism spectrum disorder. Dev Cogn Neurosci 2016;19:137–143. doi: 10.1016/j.dcn.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pote I, Wang S, Sethna V, et al. Familial risk of autism alters subcortical and cerebellar brain anatomy in infants and predicts the emergence of repetitive behaviors in early childhood. Autism Research 2019;12(4):614–627. doi: 10.1002/aur.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swanson MR, Shen MD, Wolff JJ, et al. Subcortical Brain and Behavior Phenotypes Differentiate Infants With Autism Versus Language Delay. Biol Psychiatry Cogn Neurosci Neuroimaging 2017;2(8):664–672. doi: 10.1016/j.bpsc.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fatemi SH, Aldinger KA, Ashwood P, et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012;11(3):777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Research 2009;2(5):246–257. doi: 10.1002/aur.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webb SJ, Sparks B-F, Friedman SD, et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res 2009;172(1):61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piven J, Saliba K, Bailey J, Arndt S. An MRI study of autism: the cerebellum revisited. Neurology 1997;49(2):546–551. doi: 10.1212/wnl.49.2.546. [DOI] [PubMed] [Google Scholar]
- 74.Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI Study of the Corpus Callosum and Cerebellum in Mentally Retarded Autistic Individuals. JNP 1999;11(4):470–474. [DOI] [PubMed] [Google Scholar]
- 75.Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A Two-Year Longitudinal MRI Study of the Corpus Callosum in Autism. J Autism Dev Disord 2012;42(11):2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boger-Megiddo I, Shaw DWW, Friedman SD, et al. Corpus Callosum Morphometrics in Young Children with Autism Spectrum Disorder. J Autism Dev Disord 2006;36(6):733–739. [DOI] [PubMed] [Google Scholar]
- 77.Nordahl CW, Iosif A-M, Young GS, et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol Autism 2015;6(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolff JJ, Gerig G, Lewis JD, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 2015;138(Pt 7):2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen MD, Kim SH, McKinstry RC, et al. Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants Who Later Develop Autism. Biol Psychiatry 2017;82(3):186–193. doi: 10.1016/j.biopsych.2017.02.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen MD, Nordahl CW, Li DD, et al. Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: a case-control study. The Lancet Psychiatry September 2018. doi: 10.1016/S2215-0366(18)30294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 82.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 2014;276:48–71. [DOI] [PubMed] [Google Scholar]
- 83.Travers BG, Adluru N, Ennis C, et al. Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review. Autism Research 2012;5(5):289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinstein M, Ben Sira L, Levy Y, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp 2011;32(4):534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conti E, Mitra J, Calderoni S, et al. Network over-connectivity differentiates autism spectrum disorder from other developmental disorders in toddlers: A diffusion MRI study. Hum Brain Mapp 2017;38(5):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jbabdi S, Johansen-Berg H. Tractography: Where Do We Go from Here? Brain Connectivity 2011;1(3):169–183. doi: 10.1089/brain.2011.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do“s and don”ts of diffusion MRI. Neuroimage 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 88.Fingher N, Dinstein I, Ben-Shachar M, et al. Toddlers later diagnosed with autism exhibit multiple structural abnormalities in temporal corpus callosum fibers. Cortex 2017;97:291–305. doi: 10.1016/j.cortex.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cascio C, Gribbin M, Gouttard S, et al. Fractional anisotropy distributions in 2- to 6-year-old children with autism. Journal of Intellectual Disability Research 2012;11:no–no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol 2012;12(1):9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolff JJ, Gu H, Gerig G, et al. Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. AJP 2012;169(6):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solso S, Xu R, Proudfoot J, et al. Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Cortical Function and Social Deficits in Autism 2016;79(8):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao M, Huang H, He Y. Developmental Connectomics from Infancy through Early Childhood. Trends Neurosci 2017;40(8):494–506. doi: 10.1016/j.tins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lewis JD, Evans AC, Pruett JR, et al. Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry 2014;4(5):e388–e388. doi: 10.1038/tp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis JD, Evans AC, Pruett JR, et al. The Emergence of Network Inefficiencies in Infants With Autism Spectrum Disorder. Biol Psychiatry 2017;82(3):176–185. doi: 10.1016/j.biopsych.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolff JJ, Swanson MR, Elison JT, et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol Autism 2017;8(1):8. doi: 10.1186/s13229-017-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elison JT, Paterson SJ, Wolff JJ, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naigles LR, Johnson R, Mastergeorge A, et al. Neural correlates of language variability in preschool-aged boys with autism spectrum disorder. Autism Research 2017;44:2221. doi: 10.1002/aur.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Tsang T, Jackson L, et al. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Developmental Science 2019;22(3):e12768. doi: 10.1111/desc.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry 2008;64(7):589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 2012;135(3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dinstein I, Pierce K, Eyler L, et al. Disrupted Neural Synchronization in Toddlers with Autism. Neuron 2011;70(6):1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lombardo MV, Pierce K, Eyler LT, et al. Different functional neural substrates for good and poor language outcome in autism. Neuron 2015;86(2):567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen H, Wang J, Uddin LQ, et al. Aberrant functional connectivity of neural circuits associated with social and sensorimotor deficits in young children with autism spectrum disorder. Autism Research 2018;11(12):1643–1652. doi: 10.1002/aur.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ciarrusta J, O’Muircheartaigh J, Dimitrova R, et al. Social Brain Functional Maturation in Newborn Infants With and Without a Family History of Autism Spectrum Disorder. JAMA Netw Open 2019;2(4):e191868. doi: 10.1001/jamanetworkopen.2019.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen MD, Li DD, Keown CL, et al. Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry 2016;55(9):817–824. doi: 10.1016/j.jaac.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McKinnon CJ, Eggebrecht AT, Todorov A, et al. Restricted and Repetitive Behavior and Brain Functional Connectivity in Infants at Risk for Developing Autism Spectrum Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4(1):50–61. doi: 10.1016/j.bpsc.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolff JJ, Gu H, Gerig G, et al. Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. AJP 2012;169(6):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolff JJ, Gerig G, Lewis JD, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 2015;138(Pt 7):2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen MD, Nordahl CW, Li DD, et al. Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: a case-control study. The Lancet Psychiatry September 2018. doi: 10.1016/S2215-0366(18)30294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ozonoff S, Young GS, Belding A, et al. The broader autism phenotype in infancy: when does it emerge? J Am Acad Child Adolesc Psychiatry 2014;53(4):398–407.e2. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lui JH, Hansen DV, Kriegstein AR. Development and Evolution of the Human Neocortex. Cell 2011;146(1):18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR. Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 2016;91(6):1219–1227. doi: 10.1016/j.neuron.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nature Reviews Neuroscience 2006;7(11):883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 116.Rakic P A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 1995;18(9):383–388. [DOI] [PubMed] [Google Scholar]
- 117.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016;89(2):248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Packer A Neocortical neurogenesis and the etiology of autism spectrum disorder. Neuroscience & Biobehavioral Reviews 2016;64:185–195. doi: 10.1016/j.neubiorev.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Marchetto MC, Belinson H, Tian Y, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry 2017;22(6):820–835. doi: 10.1038/mp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mariani J, Coppola G, Zhang P, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015;162(2):375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schafer ST, Paquola ACM, Stern S, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nature Neuroscience 2019;22:345. doi: 10.1038/s41593-018-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon C-H, Luikart BW, Powell CM, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron 2006;50(3):377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernier R, Golzio C, Xiong B, et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell 2014;158(2):263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deshpande A, Yadav S, Dao DQ, et al. Cellular Phenotypes in Human iPSC-Derived Neurons from a Genetic Model of Autism Spectrum Disorder. Cell Rep 2017;21(10):2678–2687. doi: 10.1016/j.celrep.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qureshi AY, Mueller S, Snyder AZ, et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J Neurosci 2014;34(34):11199–11211. doi: 10.1523/JNEUROSCI.1366-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang W-Q, Chen W-W, Jiang L, et al. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell Rep 2014;9(5):1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Suetterlin P, Hurley S, Mohan C, et al. Altered Neocortical Gene Expression, Brain Overgrowth and Functional Over-Connectivity in Chd8 Haploinsufficient Mice. Cerebral Cortex 2018;28(6):2192–2206. doi: 10.1093/cercor/bhy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Piochon C, Kano M, Hansel C. LTD-like molecular pathways in developmental synaptic pruning. Nature Neuroscience 2016;19(10):1299–1310. doi: 10.1038/nn.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hensch TK. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 130.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 2011;480(7375):63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baudouin SJ, Gaudias J, Gerharz S, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science 2012;338(6103):128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- 133.Piochon C, Kloth AD, Grasselli G, et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat Commun 2014;5(1):5586. doi: 10.1038/ncomms6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elison JT, Paterson SJ, Wolff JJ, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen MD. Cerebrospinal fluid and the early brain development of autism. J Neurodev Disord 2018;10(1):893. doi: 10.1186/s11689-018-9256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lehtinen MK, Zappaterra MW, Chen X, et al. The Cerebrospinal Fluid Provides a Proliferative Niche for Neural Progenitor Cells. Neuron 2011;69(5):893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 2008;5(1):10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4(147):147ra111–147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phan BN, Page SC, Campbell MN, et al. Defects of myelination are common pathophysiology in syndromic and idiopathic autism spectrum disorder. bioRxiv January 2017:128124. [Google Scholar]
- 140.Lee H, Thacker S, Sarn N, Dutta R, Eng C. Constitutional mislocalization of Pten drives precocious maturation in oligodendrocytes and aberrant myelination in model of autism spectrum disorder. Transl Psychiatry 2019;9(1):13. doi: 10.1038/s41398-018-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci 2010;30(44):14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nature Reviews Neuroscience 2015;16(12):756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 2019;51(3):431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Courchesne E, Pramparo T, Gazestani VH, Lombardo MV, Pierce K, Lewis NE. The ASD Living Biology: from cell proliferation to clinical phenotype. Mol Psychiatry 2018;2(Pt 9):217. doi: 10.1038/s41380-018-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ozonoff S, Young GS, Steinfeld MB, et al. How early do parent concerns predict later autism diagnosis? J Dev Behav Pediatr 2009;30(5):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chawarska K, Shic F, Macari S, et al. 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: a baby siblings research consortium study. J Am Acad Child Adolesc Psychiatry 2014;53(12):1317–1327.e1. doi: 10.1016/j.jaac.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pandey J, Verbalis A, Robins DL, et al. Screening for autism in older and younger toddlers with the Modified Checklist for Autism in Toddlers. Autism 2008;12(5):513–535. doi: 10.1177/1362361308094503. [DOI] [PubMed] [Google Scholar]
- 149.Zwaigenbaum L, Bryson S, Lord C, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics 2009;123(5):1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vieira S, Pinaya WHL, Mechelli A. Using deep learning to investigate the neuroimaging correlates of psychiatric and neurological disorders: Methods and applications. Neuroscience & Biobehavioral Reviews 2017;74:58–75. doi: 10.1016/j.neubiorev.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 151.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 2009;45(1 Suppl):S199–S209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mostapha M, Styner M. Role of deep learning in infant brain MRI analysis. Magn Reson Imaging June 2019. doi: 10.1016/j.mri.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic Minority Over-sampling Technique. 1 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 154.Taft LM, Evans RS, Shyu CR, et al. Countering imbalanced datasets to improve adverse drug event predictive models in labor and delivery. J Biomed Inform 2009;42(2):356–364. doi: 10.1016/j.jbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen MD, Piven J. Brain and behavior development in autism from birth through infancy. Dialogues Clin Neurosci 2017;19(4):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron 2015;85(1):11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Danhong W, Meiling L, Meiyun W et al. Individual-specific functional connectivity markers track dimensional and categorical features of psychotic illness. Mol Psychiatry 2018. November 15. doi: 10.1038/s41380-018-0276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang X, Gong Q, Sweeney JA, et al. Progress in psychoradiology, the clinical application of psychiatric neuroimaging. Br J Radiol 2019; 92(1101):20181000. doi: 10.1259/bjr.20181000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lei D, Pinaya WHL, van Amelsvoort T, et al. Detecting schizophrenia at the level of the individual: relative diagnostic value of whole-brain images, connectome-wide functional connectivity and graph-based metrics. Psychol Med 2019. August 8:1–10. doi: 10.1017/S0033291719001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Port JD. Diagnosis of Attention Deficit Hyperactivity Disorder by Using MR Imaging and Radiomics: A Potential Tool for Clinicians. Radiology 2018; 287: 631–632. doi: 10.1148/radiol.2018172804 [DOI] [PubMed] [Google Scholar]
- 161.Sun H, Chen Y, Huang Q, et al. Psychoradiologic Utility of MR Imaging for Diagnosis of Attention Deficit Hyperactivity Disorder: A Radiomics Analysis. Radiology. 2018. May;287(2):620–630. doi: 10.1148/radiol.2017170226 [DOI] [PubMed] [Google Scholar]
- 162.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Estes A, Munson J, Rogers SJ, Greenson J, Winter J, Dawson G. Long-Term Outcomes of Early Intervention in 6-Year-Old Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry 2015;54(7):580–587. doi: 10.1016/j.jaac.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dawson G, Rogers S, Munson J, et al. Randomized, Controlled Trial of an Intervention for Toddlers With Autism: The Early Start Denver Model. Pediatrics 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kasari C, Gulsrud A, Paparella T, Hellemann G, Berry K. Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. Journal of Consulting and Clinical Psychology 2015;83(3):554–563. doi: 10.1037/a0039080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Howlin P, Magiati I, Charman T. Systematic Review of Early Intensive Behavioral Interventions for Children With Autism MacLean WE Jr, ed. Am J Intellect Dev Disabil 2009;114(1):23–41. [DOI] [PubMed] [Google Scholar]
- 167.Whitehouse AJO, Varcin KJ, Alvares GA, et al. Pre-emptive intervention versus treatment as usual for infants showing early behavioural risk signs of autism spectrum disorder: a single-blind, randomised controlled trial. The Lancet Child & Adolescent Health 2019;3(9):605–615. doi: 10.1016/S2352-4642(19)30184-1. [DOI] [PubMed] [Google Scholar]
- 168.Girault JB, Munsell BC, Puechmaille D, et al. White matter connectomes at birth accurately predict cognitive abilities at age 2. Neuroimage 2019;192:145–155. doi: 10.1016/j.neuroimage.2019.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kawahara J, Brown CJ, Miller SP, et al. BrainNetCNN: Convolutional neural networks for brain networks; towards predicting neurodevelopment. Neuroimage 2017;146:1038–1049. doi: 10.1016/j.neuroimage.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 170.Constantino JN. Early behavioral indices of inherited liability to autism. Pediatr Res 2018;114(5 Pt 2):129. doi: 10.1038/s41390-018-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Constantino JN, Charman T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol 2016;15(3):279–291. doi: 10.1016/S1474-4422(15)00151-9. [DOI] [PubMed] [Google Scholar]
- 172.Feczko E, Balba NM, Miranda-Dominguez O, et al. Subtyping cognitive profiles in Autism Spectrum Disorder using a Functional Random Forest algorithm. Neuroimage 2018;172:674–688. doi: 10.1016/j.neuroimage.2017.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gates KM, Molenaar PCM, Iyer SP, Nigg JT, Fair DA. Organizing Heterogeneous Samples Using Community Detection of GIMME-Derived Resting State Functional Networks Zhou J, ed. PLoS ONE 2014;9(3):e91322. doi: 10.1371/journal.pone.0091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gordon EM, Laumann TO, Adeyemo B, et al. Individual-specific features of brain systems identified with resting state functional correlations. Neuroimage 2017;146:918–939. doi: 10.1016/j.neuroimage.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gates KM, Molenaar PCM. Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. Neuroimage 2012;63(1):310–319. doi: 10.1016/j.neuroimage.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 176.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 177.Jacob S, Wolff JJ, Steinbach MS, Doyle CB, Kumar V, Elison JT. Neurodevelopmental heterogeneity and computational approaches for understanding autism. Transl Psychiatry 2019;9(1):63. doi: 10.1038/s41398-019-0390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nat Genet 2014;46(8):881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell 2017;169(7):1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weiner DJ, Wigdor EM, Ripke S, et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet 2017;49(7):978–985. doi: 10.1038/ng.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moreno-De-Luca A, Evans DW, Boomer KB, et al. The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11.2 deletions. JAMA Psychiatry 2015;72(2):119–126. doi: 10.1001/jamapsychiatry.2014.2147. [DOI] [PubMed] [Google Scholar]
- 182.LeBarton ES, Landa RJ. Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behavior and Development 2019;54:37–47. doi: 10.1016/j.infbeh.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 183.Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development 2012;35(4):838–846. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research 2016;9(9):993–1001. doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gopalakrishnan J The Emergence of Stem Cell-Based Brain Organoids: Trends and Challenges. Bioessays 2019;41(8):e1900011. doi: 10.1002/bies.201900011. [DOI] [PubMed] [Google Scholar]