Fig 1. In membrane-embedded translocon pores, individual IpaC molecules are adjacent to one another.
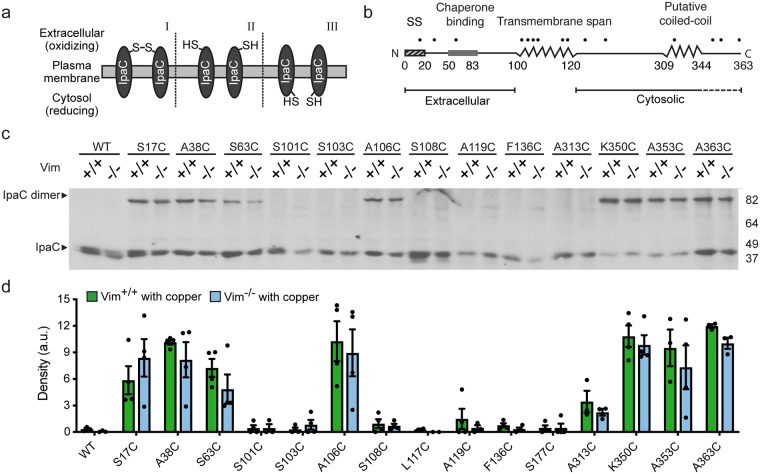
(a) Schematic of the dependence of disulfide bond formation on an oxidative environment and the relative positioning of free sulfhydryl groups. Disulfide bond formation occurs when residues are located in the extracellular space, and when in close proximity with the correct orientation of the residues in adjacent molecules [I]. The formation of disulfide bonds is not efficient when residues are in an oxidative environment but are not in proximity or correctly oriented [II], or when residues are in a reducing environment, such as the cytosol [III]. (b) Schematic of secondary structure and orientation in the membrane of IpaC protein. Dots indicate the relative position of the mutants tested. Dashed line beneath residues 344–363 reflects the accessibility of these residues to the extracellular milieu [23], suggesting that they loop into the pore lumen. (c-d) Analysis of intermolecular crosslinking of single cysteine substitutions of IpaC delivered to cells during S. flexneri infection. Infection of Vim+/+ or Vim-/- MEFs in the presence of the oxidant copper with strains of S. flexneri ΔipaC producing individual IpaC cysteine substitutions. Representative western blot of IpaC, showing bands migrating at the molecular weight of IpaC dimers and monomers (c). Densitometry of the ~80 kilodalton band from three to four independent experiments; mean ± SEM (d). For cysteine substitution derivatives, band densities are not significantly different for infection of Vim+/+ versus Vim-/- MEFs.