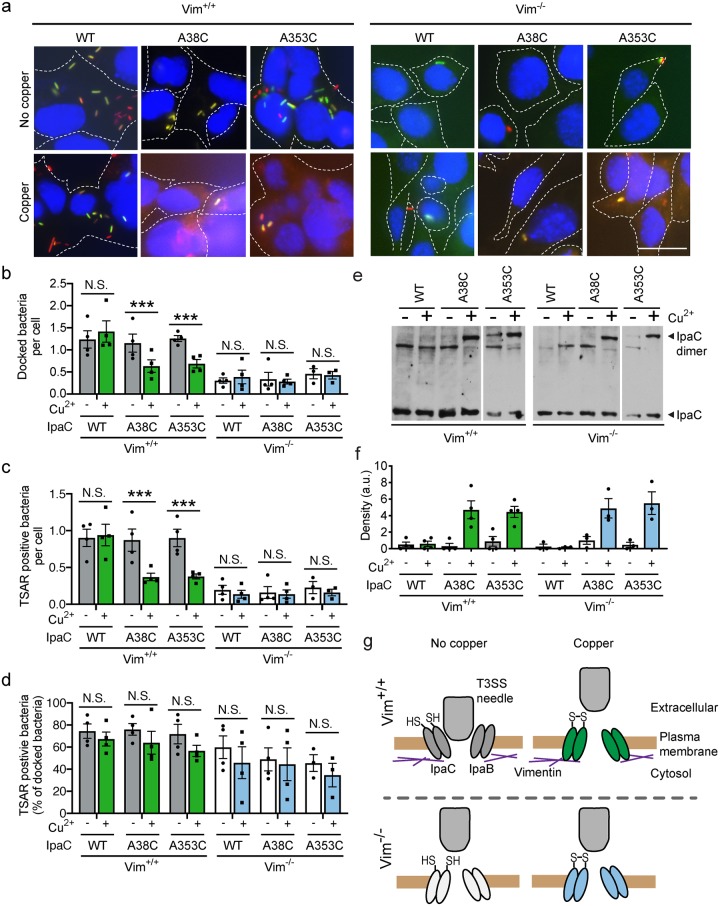
Fig 2. Intermolecular crosslinking of IpaC molecules in the translocon pore at the time of initial bacterial contact with cells inhibits docking.
Efficiency of docking and T3SS-mediated secretion upon addition of the oxidant copper during S. flexneri infection of Vim+/+ or Vim-/- MEFs. Copper was added at the time of initial bacterial contact with cells. IpaC or its derivatives were expressed in S. flexneri ΔipaC containing the TSAR reporter of T3SS secretion [37]. (a) Representative micrographs of S. flexneri-infected cells, imaged at 50 minutes of infection. Blue, DNA; red, mCherry (all bacteria); green, GFP (bacteria actively secreting through T3SS). Scale bar, 20 μM. (b) Efficiency of docking of bacteria on cells. (c-d) The number of docked bacteria per cell (c) and the percentage of docked bacteria that are actively secreting (d), as indicated by TSAR reporter, from images in experiments represented in panel a. (e) IpaC dimer formation analyzed at 20 minutes of infection. Non-reducing western blot of IpaC, representative of three independent experiments; all panels are from the same blot. (f) Densitometry analysis of bands corresponding to IpaC dimers in experiments represented in panel e. (g) Model for the effect of IpaC crosslinking on restricting shifting of the translocon pore in the plasma membrane. Graphed data are presented as mean ± SEM of three to four independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001; two-way ANOVA with Sidak’s post hoc test (b-d).