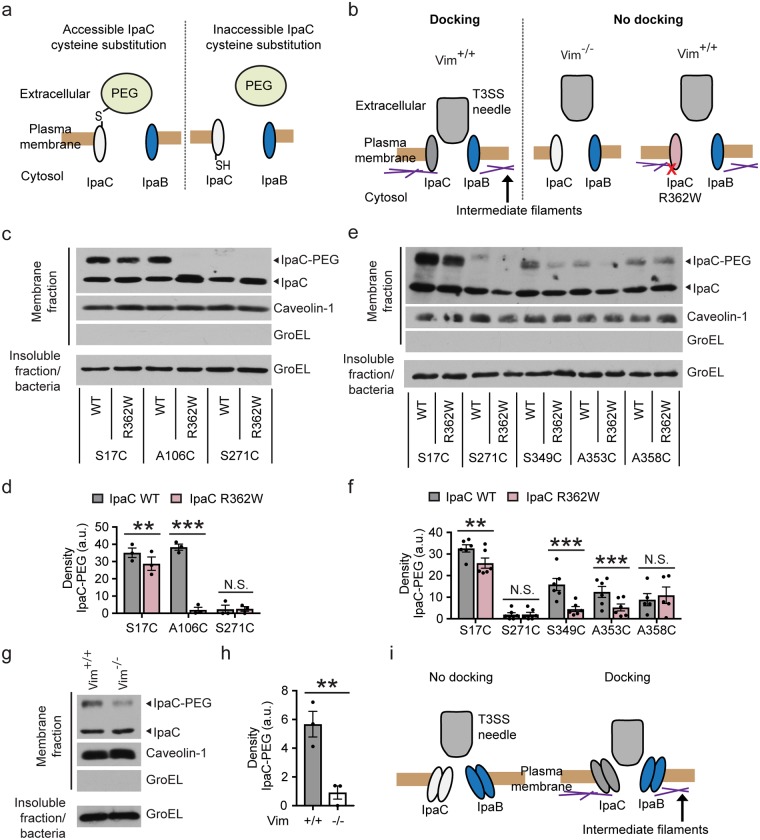
Fig 3. Accessibility of IpaC residues from the extracellular surface is altered by the interaction of IpaC with intermediate filaments.
(a) Schematic of PEG5000-maleimide labeling of IpaC residues accessible from the extracellular face of the plasma membrane. PEG, PEG5000-maleimide. (b) Schematic of docking phenotype for translocon pores formed by WT IpaC or IpaC R362W, a derivative of IpaC unable to interact with intermediate filaments. (c-f) PEG5000-maleimide labeling of sulfhydryl groups in cysteine substitution derivatives in the context of WT IpaC or IpaC R362W during S. flexneri infection of HeLa cells. PEG5000-maleimide labeling of residue A106C in the transmembrane span (c-d) and residues S349C and A358C near the C terminus (e-f). Representative western blot of IpaC in plasma membrane-enriched fractions following PEG5000-maleimide labeling during infection (c and e). Densitometry analysis of bands corresponding to IpaC-PEG5000 (d and f). IpaC-PEG5000, IpaC derivatives labeled with PEG5000-maleimide; IpaC, unlabeled IpaC derivatives. (g-h) Decreased accessibility of IpaC A106C during infection of cells lacking intermediate filaments (Vim-/-) as compared with cells containing intermediate filaments (Vim+/+). Representative western blot of IpaC in plasma membrane-enriched fractions following PEG5000-maleimide labeling during infection with S. flexneri producing IpaC A106C (g). Caveolin-1, marker of eukaryotic plasma membrane; GroEL, bacterial cytosolic protein. Densitometry analysis of IpaC-PEG5000 bands (h). (i) Model of the dependence of docking of the T3SS on conformational changes in the translocon pore induced by the interaction of IpaC with intermediate filaments. Graphed data are presented as mean ± SEM of three independent experiments. Two-way ANOVA with a Sidak post hoc test (d and f) or Student’s t-test (h). **, p<0.01; ***, p<0.001, N.S., not significant.