Spores are required for long-term survival of many organisms, including most fungi. For the majority of fatal human fungal pathogens, spore germination is the key process required to initiate vegetative growth and ultimately cause disease. Because germination is required for pathogenesis, the process could hold fungus-specific targets for new antifungal drug development.
KEYWORDS: fungal spores, germination inhibitors, antifungal drugs, drug repurposing, primary prophylaxis, Cryptococcus
ABSTRACT
Spores are required for long-term survival of many organisms, including most fungi. For the majority of fatal human fungal pathogens, spore germination is the key process required to initiate vegetative growth and ultimately cause disease. Because germination is required for pathogenesis, the process could hold fungus-specific targets for new antifungal drug development. Compounds that inhibit germination could be developed into high-efficacy, low-toxicity drugs for use in the prevention and/or treatment of fungal spore-mediated diseases. To identify drugs with the ability to inhibit pathogenic fungal spore germination, we developed a novel luciferase-based germination assay, using spores of the meningitis-causing yeast Cryptococcus. We screened the L1300 Selleck Library of U.S Food and Drug Administration-approved drugs and identified 27 that inhibit germination. Of these, 22 inhibited both germination and yeast growth, and 21 have not been previously indicated for use in the treatment of fungal diseases. We quantitated the inhibition phenotypes of 10 specific germination/growth inhibitors in detail and tested one drug, the antiparasitic compound pentamidine, in our mouse intranasal model of cryptococcal infection. We discovered that pentamidine was effective at reducing lung fungal burdens when used in either prophylaxis (before infection) or treatment (after establishing an infection). Due to its efficacy in vivo and low intranasal toxicity, pentamidine is a lead candidate for repurposing for broader use as an antigerminant to prevent spore-mediated disease in immunocompromised patients. Not only does pentamidine provide an opportunity for prophylaxis against fungal spores, but it also provides proof of concept for targeting pathogenic spore germination for antifungal drug development.
INTRODUCTION
Fungal diseases affect over a billion people, resulting in ∼1.5 million deaths annually (1). Currently, there are three primary classes of antifungal drugs used to treat invasive fungal disease, and all of them are subject to limitations (2). Acute drug toxicity in humans, the emergence of fungal drug resistance, and insufficient efficacy have resulted in a relatively weak arsenal to combat fungal infections (2–5). Novel antifungal agents need to be developed; however, due to the shared eukaryotic nature of fungi and humans, compounds that inhibit fungi are often also toxic to humans, and this is one of the main hurdles in antifungal drug development. In the past, a common strategy for minimizing drug toxicity was to inhibit two known fungus-specific pathways: fungal cell wall synthesis or ergosterol metabolism (2, 6). This limited number of targets has contributed, in part, to the limited number of antifungal drugs. To expand the number of potential targets for new antifungal drugs, another strategy could be to inhibit fungus-specific developmental processes more broadly.
One process that could be targeted for antifungal development is fungal spore germination. Spores are dormant, stress-resistant cell types that are known to cause disease among both plant and animal fungal pathogens (7, 8). Spores cannot reproduce and must differentiate (germinate) to grow vegetatively. Because spore-mediated disease results from overwhelming fungal growth, spore germination is an essential process for spore-mediated disease. Relative to other fungal processes, germination is poorly understood, particularly among human fungal pathogens; however, it appears distinct from processes that occur in humans, suggesting that spore germination involves fungus-specific cellular pathways (7). Due to its fungal specificity and importance in spore-mediated disease, we hypothesize that fungal spore germination and the molecular processes involved are ideal targets for the development of novel antifungal drugs.
Because the timeline for novel drug development can be decades and the need for new antifungal drugs is acute, we prioritized the evaluation of existing U.S. Food and Drug Administration (FDA)-approved drugs for repurposing. These drugs have already passed toxicity testing in humans, allowing them to reach patients in need sooner than other potential therapeutics (9). In this study, we used a high-throughput assay to screen a library of off-patent FDA-approved drugs to identify those that inhibit the human fungal pathogen Cryptococcus neoformans. Cryptococcus is the most common cause of fungal meningitis and has been established as a research model for human fungal pathogens. This environmental basidiomycete yeast undergoes sexual development (both same-sex and opposite-sex) to form basidiospores (10). Both yeast and spores are proposed infectious particles in human disease (11–14). During germination, spores transition from small, ovoid particles to large, circular yeast, which then engage in clonal growth via budding (15). Using Cryptococcus spore germination as a novel screening target, we discovered and characterized ten existing FDA-approved drugs that inhibited germination.
One of these newly discovered antigerminant drugs, the aromatic diamidine compound pentamidine was chosen for in vivo characterization due to its high potential for repurposing/expanded use against fungi. Because the route of infection by pathogenic fungal spores is generally through inhalation (16), the use of aerosolized pentamidine in prophylaxis against fatal fungal diseases could be an effective strategy. To determine the ability of pentamidine to prevent fungal spore-mediated disease in vivo, we evaluated its antifungal and antigermination activity in a murine model of spore-derived cryptococcosis. We discovered that pentamidine was tolerated well by the mice and exhibited antifungal and antigerminant activity, resulting in significantly decreased cryptococcal lung burdens. Taken together, our data support the hypothesis that spore germination is a viable target for antifungal drug development, and existing drugs may play a role in preventing and treating invasive fungal diseases.
RESULTS
High-throughput screening for inhibitors of yeast growth and spore germination identified ten drug candidates for repurposing.
To identify inhibitors of Cryptococcus spore germination and yeast growth, we screened the L1300 Selleck FDA-Approved Drug Library containing an array of 1,018 compounds (purchased from Selleck Chemicals). Knowing that spores become more sensitive to cell lysis as they germinate into yeast, we created a reporter strain in which a protein known to be present in spores (Isp4) was fused to the luciferase gene NanoLuc (Promega Corporation). Spores containing the construct were not very susceptible to lysis and produced low levels of nanoluciferase (NL) signal in the NanoLuc assay. As they germinated into yeast, they became more sensitive to lysis, producing higher nanoluciferase signals. In response to germinating conditions, the luciferase levels increased ∼20-fold after 10 h of incubation at 30°C (full germination into yeast) and remained high thereafter. Thus, there was a direct correlation between luciferase activity and state of germination, allowing the use of luciferase activity as a marker of germination progression. This screen was coupled with optical density at 600 nm (OD600) readings to monitor the ability of compounds to also inhibit yeast growth.
For the purposes of this study, we defined known antifungal drugs as any FDA drug approved for use in the treatment of fungal infections. We predicted that our growth assays would identify the vast majority of known antifungal drugs in the library, and this was the case. Using a cutoff for growth inhibition defined as an OD600 signal of <75% of the no-drug control OD600, we detected 23 of 24 known antifungal drugs (Table 1). The exception was flucytosine, which is among the least effective of designated antifungal drugs. Lack of detection in the screen was possibly due to the concentration of flucytosine (1.3 μg/ml) being below the MIC required for inhibition of the JEC20/21 strain background (17). Using a cutoff for germination inhibition defined as a luciferase signal of <30% of the no-drug control signal, we identified six germination inhibitors among the 24 antifungal drugs (highlighted in Table 1).
TABLE 1.
Inhibitory activity of known antifungal drugs against Cryptococcus yeast growth and spore germinationa
| Known antifungal drugs | Yeast growth (%OD600) | Spore germination (% luciferase signal) |
|---|---|---|
| No drug | 100.0 | 100.0 |
| Butenafine HCl | 32.9 | 57.5 |
| Econazole nitrate | 33.1 | 16.1 |
| Bifonazole | 33.4 | 13.6 |
| Butoconazole nitrate | 33.4 | 41.5 |
| Clotrimazole | 34.1 | 65.9 |
| Naftifine HCl | 34.5 | 55.5 |
| Climbazole | 34.8 | 151.5 |
| Ketoconazole | 35.4 | 154.8 |
| Voriconazole | 35.8 | 173.9 |
| Fenticonazole nitrate | 36.4 | 49.1 |
| Tioconazole | 36.8 | 25.0 |
| Isoconazole nitrate | 37.0 | 16.8 |
| Liranaftate | 37.6 | 64.8 |
| Miconazole nitrate | 38.2 | 25.5 |
| Pentamidine HCl | 38.3 | 6.5 |
| Sulconazole nitrate | 40.9 | 57.8 |
| Amorolfine hydrochloride | 41.4 | 88.6 |
| Amphotericin B | 45.7 | 84.6 |
| Posaconazole | 46.7 | 167.2 |
| Tolnaftate | 47.3 | 60.2 |
| Caspofungin acetate | 48.5 | 89.7 |
| Itraconazole | 58.9 | 159.6 |
| Fluconazole | 72.2 | 84.0 |
| Flucytosine | 96.4 | 102.0 |
Inhibitors of spore germination are indicated by gray shading.
Using the same cutoff values for nanoluciferase signal (<30% of the control) and OD600 (<75% of the control), we identified an additional 60 inhibitors of yeast growth among the drugs in the library, 16 of which also inhibited germination (Table 2). Five drugs inhibited only germination (and not vegetative growth). Because it is unlikely that inhibition of a population of spores would be 100% efficient, we prioritized drugs that were inhibitors of both germination and growth so that any spores that escape germination inhibition would also be prevented from growing vegetatively.
TABLE 2.
Inhibitory activity of known antifungal drugs against Cryptococcus yeast growth and spore germination, with designations as listed by the L1300 Selleck FDA-approved drug librarya
| Drug | Yeast growth (%OD600) | Spore germination (% luciferase signal) | Designation |
|---|---|---|---|
| Otilonium bromide | 29.0 | 6.9 | Cardiovascular disease |
| Alexidine HCl | 29.6 | 4.6 | |
| Benzethonium chloride | 30.3 | 6.9 | Neurological disease |
| Clomifene citrate | 30.7 | 66.0 | Cancer |
| Tamoxifen citrate | 31.4 | 71.7 | Endocrinology |
| Cetylpyridinium chloride | 31.9 | 4.2 | Infection |
| Mizolastine | 32.7 | 144.5 | |
| Terbinafine | 32.9 | 54.3 | Infection |
| Arecoline | 33.2 | 79.5 | Endocrinology |
| 10-DAB | 34.6 | 104.1 | Cancer |
| Irbesartan | 34.9 | 180.8 | |
| Vincristine | 35.5 | 99.7 | Cancer |
| Chloroxine | 35.9 | 41.9 | Infection |
| Rivastigmine tartrate | 36.5 | 99.4 | Cardiovascular disease |
| Betaxolol | 37.1 | 102.5 | Cardiovascular disease |
| AMG-073 HCl | 37.5 | 53.2 | Endocrinology |
| Cyproheptadine HCl | 37.8 | 90.7 | Neurological disease |
| Toremifene citrate | 38.1 | 80.3 | Endocrinology |
| Cytidine | 38.4 | 114.9 | Cardiovascular disease |
| Mexiletine HCl | 39.0 | 98.3 | Cardiovascular disease |
| Azasetron HCl | 39.1 | 98.4 | |
| Zalcitabine | 40.1 | 96.5 | Infection |
| Primidone | 42.3 | 99.5 | Neurological disease |
| Isradipine | 42.7 | 66.7 | Neurological disease |
| Epalrestat | 42.9 | 68.8 | Inflammation |
| Niclosamide | 43.4 | 7.8 | |
| Metoprolol tartrate | 43.5 | 97.3 | Cardiovascular disease |
| Dasatinib | 44.7 | 53.3 | Cancer |
| Besifloxacin HCl | 44.7 | 123.4 | |
| Broxyquinoline | 46.0 | 79.6 | Vermifuge |
| Daptomycin | 46.0 | 98.2 | |
| Metolazone | 46.7 | 93.6 | Cardiovascular disease |
| Dequalinium chloride | 47.9 | 22.3 | |
| Hygromycin B | 49.2 | 72.4 | |
| Cefdinir | 50.0 | 80.4 | Infection |
| Ezetimibe | 51.3 | 22.0 | Cardiovascular disease |
| Benazepril HCl | 52.6 | 39.4 | Cardiovascular disease |
| Doxercalciferol | 55.2 | 17.1 | Endocrinology |
| Methimazole | 55.9 | 97.4 | Endocrinology |
| Deferiprone | 56.2 | 68.3 | |
| Biperiden HCl | 56.6 | 23.4 | Neurological disease |
| Alverine citrate | 56.8 | 78.2 | Digestive system disease |
| Tolfenamic acid | 58.9 | 71.4 | Inflammation |
| Temsirolimus | 59.2 | 21.3 | Cancer |
| Ethambutol HCl | 61.3 | 92.5 | Neurological disease |
| Rapamycin | 61.8 | 18.5 | Immunology |
| Cetrimonium bromide | 63.2 | 4.4 | Infection |
| Domiphen bromide | 63.9 | 4.4 | Infection |
| Disulfiram | 65.6 | 22.7 | Neurological disease |
| Mycophenolate mofetil | 65.8 | 51.0 | Immunology |
| Famciclovir | 66.6 | 80.2 | Cancer |
| Everolimus | 67.7 | 15.7 | Cancer |
| Metformin HCl | 67.9 | 96.5 | |
| Entacapone | 69.0 | 75.2 | Neurological disease |
| Sasapyrine | 69.6 | 84.3 | Inflammation |
| PCI-32765 | 70.3 | 10.6 | Neurological disease |
| Allylthiourea | 70.9 | 69.5 | Metabolic disease |
| Imidapril | 73.0 | 110.7 | Cardiovascular disease |
| Riluzole | 73.7 | 85.4 | Neurological disease |
| Disodium cromoglycate | 74.9 | 93.8 | |
| Sulfanilamide | 87.5 | 21.0 | Infection |
| Ethinyl estradiol | 93.1 | 20.2 | Endocrinology |
| Candesartan cilexetil | 99.4 | 28.8 | Cardiovascular disease |
| Triclabendazole | 106.0 | 9.10 | Vermifuge |
| Aripiprazole | 110.0 | 21.3 | Neurological disease |
Inhibitors of spore germination are indicated by dark-gray shading. Inhibitors of germination only are indicated by light-gray shading.
In total, through this screen of FDA-approved drugs, we identified 22 drugs with both germination inhibition and growth inhibition capacities. Based on designated categories of current use and dose-response curves, we chose 10 inhibitors (3 known antifungal drugs and 7 additional drugs) for further analyses (Table 3). All 10 drugs produced nanoluciferase signal-derived concentration responses that were best fit to monotonic, sigmoid curves, consistent with standard drug dose-response models (see Fig. S1 in the supplemental material) (18). In all cases, the data are consistent with a causal relationship between germination inhibition (as determined by the luciferase signal) and increasing exposure to drug.
TABLE 3.
Inhibitory activity of FDA approved drugs against spore germination and yeast replicationa
| Drug | Germination (% luciferase signal) | Yeast growth (%OD600) | Uses |
|---|---|---|---|
| Pentamidine HCl | 6.5 | 38.3 | Pneumocystis pneumonia treatment |
| Bifonazole | 13.6 | 33.4 | Cutaneous fungal infection treatment |
| Econazole nitrate | 16.1 | 33.1 | Cutaneous fungal infection treatment |
| Cetylpyridinium chloride | 4.2 | 31.9 | Antimicrobial oral care |
| Alexidine HCl | 4.6 | 29.6 | Antimicrobial oral care |
| Otilonium bromide | 6.9 | 29.0 | Irritable bowel syndrome treatment |
| Benzethonium chloride | 6.9 | 30.3 | Antimicrobial oral care |
| Niclosamide | 7.8 | 43.4 | Helminth infection treatment |
| Temsirolimus | 21.3 | 59.2 | Cancer treatment |
| Disulfiram | 22.7 | 65.6 | Alcoholism treatment |
A list of drugs and their abilities to inhibit Cryptococcus spore germination (based on luciferase signal) and yeast replication (based on OD600), as well as a brief description of their primary uses, is provided. Known antifungal drugs are highlighted by gray shading.
Validation and quantitation of antifungal activities of ten drug candidates for repurposing.
To validate the high-throughput screen results for our top 10 candidates, we determined MICs and minimal fungicidal concentrations (MFCs) for each of them in independent growth assays (Table 4). We tested them against the three most common causes of fatal fungal disease in humans (Cryptococcus neoformans, Aspergillus fumigatus, and Candida albicans), which represent phylogenetically and biologically diverse fungal pathogens. Using standard Clinical and Laboratory Standards Institute (CLSI) and EUCAST methodologies (19, 20), we determined that two of the three previously known antifungal drugs (pentamidine and bifonazole) were highly effective against two common serotypes of Cryptococcus with MICs of ≤6.25 μg/ml with primarily fungicidal activity but showed poor activity against Candida and Aspergillus. In contrast, the third known antifungal drug (econazole) was highly effective at both preventing growth and killing all of the fungi tested, with MICs and MFCs of ≤6.25 and ≤12.5 μg/ml, respectively. These findings were generally consistent with previously published data about the antifungal properties of these drugs with the exception of pentamidine, which produced significantly lower MFCs against Cryptococcus than previously reported (21–23).
TABLE 4.
Inhibitory activity of FDA approved drugs against diverse invasive fungia
| Drug | MIC or MFC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
|
Cryptococcus neoformans (JEC21) |
Cryptococcus neoformans (H99) |
Candida albicans (SC5314) |
Aspergillus fumigatus (AF293) |
||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC only | |
| Pentamidine isethionate | 1.56 | 3.13 | 6.25 | 6.25 | 50 | 50 | >100 |
| Bifonazole | 6.25 | 6.25 | 6.25 | >100 | >100 | >100 | >100 |
| Econazole nitrate | <0.78 | 6.25 | <0.78 | 6.25 | 6.25 | 12.5 | 3.13 |
| Cetylpyridinium chloride | <0.78 | <0.78 | <0.78 | <0.78 | 1.56 | 3.13 | 1.56 |
| Alexidine HCl | <0.78 | <0.78 | <0.78 | <0.78 | <0.78 | <0.78 | <0.78 |
| Otilonium bromide | 3.13 | 3.13 | 3.13 | 3.13 | 3.13 | 3.13 | 6.25 |
| Benzethonium chloride | 3.13 | 3.13 | 3.13 | 3.13 | 6.25 | 12.5 | 12.5 |
| Niclosamide | <0.78 | 1.56 | 1.56 | >100 | >100 | >100 | >100 |
| Temsirolimus | 6.25 | 6.25 | 6.25 | 6.25 | 1.56 | 1.56 | >100 |
| Disulfiram | 3.13 | 3.13 | 6.25 | 6.25 | 6.25 | 12.5 | 25 |
Known antifungal drugs are highlighted by gray shading.
The seven new drugs from the screen included diverse drugs used in antimicrobial mouthwashes (cetylpyridinium, alexidine, and benzethonium), treatments for irritable bowel syndrome (IBS) and tapeworm infections (otilonium and niclosamide, respectively), an mTOR pathway inhibitor (temsirolimus), and an acetaldehyde dehydrogenase inhibitor (disulfiram). We determined the MICs and MFCs for each of them and found that they all showed excellent activity against Cryptococcus, with MICs of ≤6.25 μg/ml with primarily fungicidal activity. All of the drugs except niclosamide showed excellent activity against Candida, and all but niclosamide, temsirolimus, and disulfiram were highly active against Aspergillus. These data confirm the findings of the initial screen for growth inhibition of Cryptococcus and confirm or establish antifungal activities for these drugs against both Candida and Aspergillus.
Validation and quantitation of antigerminant activities of ten drug candidates for repurposing.
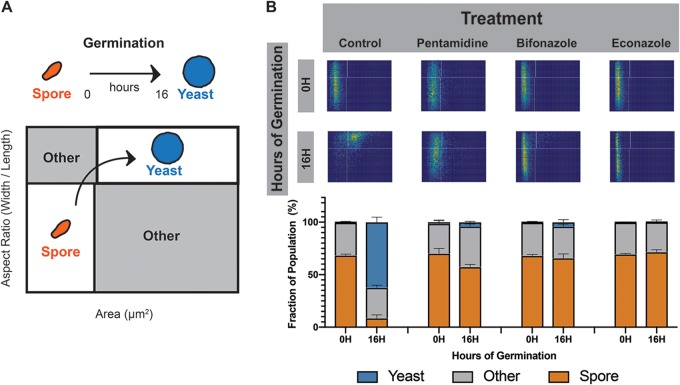
To quantitate the efficacy of the 10 drugs to inhibit Cryptococcus spore germination, we used a high-resolution microfluidics-based germination assay. In this assay, ∼10,000 purified spores per condition are loaded into a microdevice in the presence of nutrients and monitored microscopically for changes in size and shape (15). This process is monitored over 16 h at 30°C and can be used to quantify the fraction of spores and yeast at different time points and monitor the process of germination (Fig. 1A). We carried out this assay in the presence of no drug or 25 μg/ml pentamidine, bifonazole, or econazole and assessed the cell populations at 0 and 16 h. We observed that the control spore population incubated without added drug transitioned fully from spores into yeast, whereas the spores incubated in the presence of any of the three known antifungal drugs did not transition into yeast and remained small and ovoid (Fig. 1B, top panel). Quantitation of the cell populations in each case confirmed that the no-drug control cells transitioned from a population of nearly all spores into a population of nearly all yeast. In contrast, spores in the presence of the drugs remained almost entirely spores with only a small percentage (<5%) transitioning into yeast in the cases of pentamidine and bifonazole and no detectable transition in the presence of econazole (Fig. 1B, bottom panel). These data validate the nanoluciferase germination assay results and also establish three previously known inhibitors of fungal growth as bona fide inhibitors of spore germination.
FIG 1.
Antifungal drugs that are potent inhibitors of spore germination. (A) Diagram of spore germination plots. Spores, which are small and oval with aspect ratios (AR) of <0.8, appear in the lower left quadrant of two-dimensional histograms. When exposed to germinants, the spores gradually become more circular, with ARs approaching 1 and larger as they germinate until they develop fully into yeast and appear in the upper right quadrant of the plot. This process is used to quantify the fraction of spores and yeast at different time points and to monitor the process of germination. (B) Germination profiles of spores (JEC20×JEC21) exposed to no drug, 25 μg/ml pentamidine, 25 μg/ml bifonazole, or 25 μg/ml econazole at 0 and 16 h of germination (top panel). Each germination profile has an aspect ratio going from 0.4 to 1 on the y axis and an area going from 0 to 25 μm2 on the x axis. Quantification of cell populations at both time points for each condition is displayed in bar plots (bottom panel). Each profile contains roughly 10,000 individual cells.
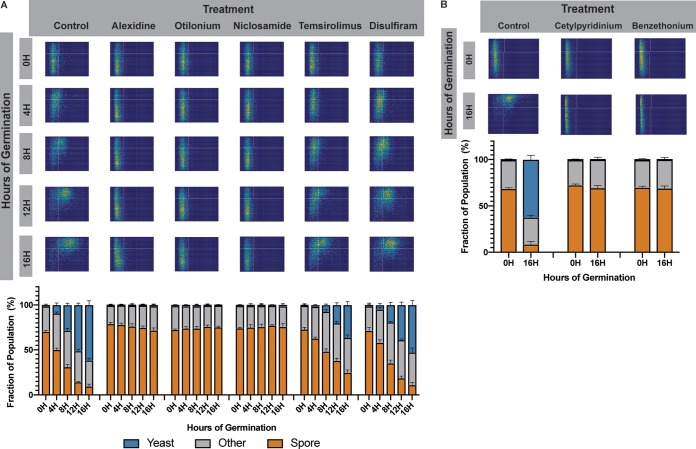
Five of the seven remaining drugs from the screen were also tested in the microfluidic devices to determine their germination inhibition phenotypes. As before, ∼10,000 spores per condition were loaded into microdevices in the presence of nutrients and treated with either no drug or 25 μg/ml alexidine, otilonium, niclosamide, temsirolimus, or disulfiram. Devices were incubated at 30°C and assessed microscopically at 0, 4, 8, 12, and 16 h. Consistent with our high-throughput screen results, we observed that all of the drugs tested in this assay were inhibitors of spore germination to various degrees (Fig. 2A). Alexidine hydrochloride, otilonium bromide, and niclosamide were all able to completely inhibit spore germination, as seen by the lack of change in spore morphology between 0 and 16 h (Fig. 2A, columns 2 to 4). In contrast, temsirolimus at this concentration appeared to only slow down the process of germination overall. When spores were exposed to temsirolimus, they initiated and maintained germination, but at a slower rate, leading to a delay in both transitioning from ovoid to round and growing in size (Fig. 2A, column 5). This observation was supported by the quantitation shown in stacked bar plots (Fig. 2A, column 5, bottom). In temsirolimus the cell populations at 16 h much more closely resembled those at 8 h in the no-drug control, indicating an ∼8-h lag in germination. Finally, disulfiram was a weak inhibitor of germination at this concentration, leading to an ∼2-h lag in germination overall. At higher concentrations of disulfiram (40 μg/ml), spores appear to stall at ∼8 h, unable to increase in size but recover by 20 h (Fig. S2).
FIG 2.
FDA drugs that are potent inhibitors of spore germination. (A) Germination profiles of spores (JEC20×JEC21) exposed to 0 or 25 μg/ml of alexidine, otilonium bromide, niclosamide, temsirolimus, or disulfiram in germinating conditions at 0, 4, 8, 12, and 16 h of germination (top panel). Each germination profile has an aspect ratio going from 0.4 to 1 on the y axis and an area going from 0 to 25 μm2 on the x axis. Quantification of the cell population at each time point was determined for each condition displayed in bar plots (bottom panel). Each profile represents ∼10,000 individual cells. (B) Germination profiles of spores (JEC20×JEC21) exposed to no drug, 25 μg/ml cetylpyridinium chloride, or 25 μg/ml benzethonium chloride at 0 and 16 h (top panel). Each germination profile has an aspect ratio going from 0.4 to 1 on the y axis and an area going from 0 to 25 μm2 on the x axis. Quantification of cell populations at both time points for each condition is displayed in bar plots (bottom panel).
The two remaining drugs, cetylpyridinium chloride and benzethonium chloride, could not be tested in the microfluidic devices due to their viscosity and were therefore tested in a modified larger-volume assay and imaged at only 0 and 16 h. Both drugs were able to fully inhibit spore germination (Fig. 2B). Taken together, these assays confirm that we identified seven FDA-approved drugs not typically used as antifungal agents that are able to inhibit fungal spore germination, leading to the possibility of repurposing these drugs for uses in the prevention of fatal fungal diseases.
The antimicrobial drug pentamidine uniformly slows and then halts germination.
Spore-producing human fungal pathogens generally infect their hosts via inhalation, making the lungs the best target for prophylaxis against infectious spores. Of the 10 drugs identified with both antigrowth and antigerminant activity, pentamidine was the most well characterized in terms of its utility in the human lung. Pentamidine is an antiparasitic drug currently approved for both prophylaxis and treatment of a single fungal pathogen, Pneumocystis jeroveci, which causes Pneumocystis pneumonia in immunocompromised patients (and was originally thought to be a protozoan parasite) (24, 25). Pentamidine exists in an aerosolized formulation that leads to a build-up of the drug in the lungs over time with limited toxicity (26, 27). Its efficacy against Cryptococcus growth and germination make it an ideal candidate for further testing and characterization as a fungal antigerminant.
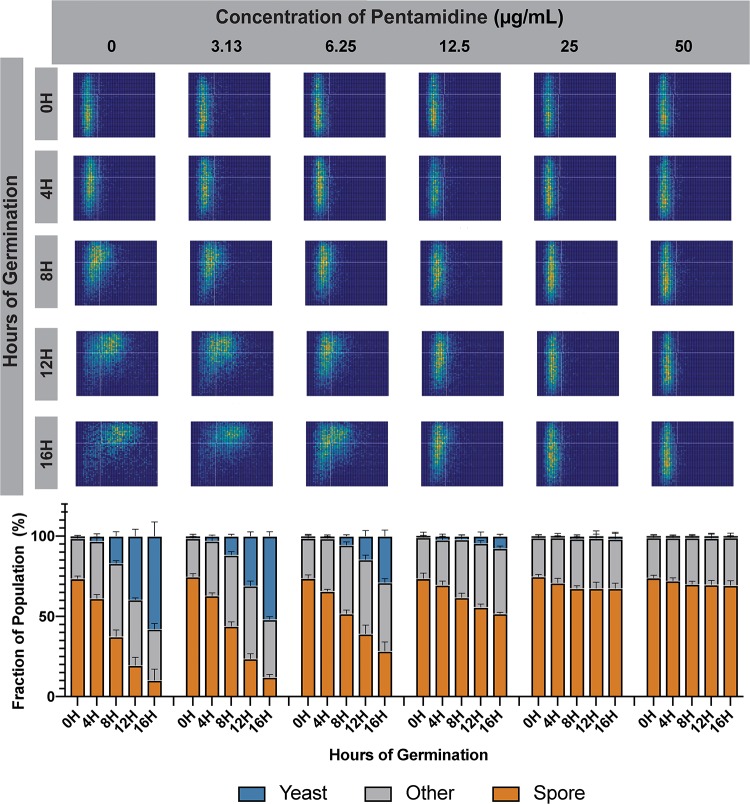
To determine a complete phenotype profile for pentamidine in germination inhibition, we evaluated a wide range of concentrations of pentamidine isethionate in the microfluidics-based germination assay. In this experiment ∼10,000 spores per condition were loaded into microdevices in the presence of nutrients and treated with either 0, 3.13, 6.25, 12.5, 25, or 50 μg/ml of pentamidine. Devices were incubated at 30°C and assessed microscopically at 0, 4, 8, 12, and 16 h. As anticipated from our initial analysis (Fig. 1), pentamidine inhibited germination completely at 25 μg/ml (Fig. 3). Pentamidine also showed inhibition of germination in a concentration-dependent manner. Minor inhibition occurred at 3.13 μg/ml, resulting in an ∼2-h delay over the course of germination (Fig. 3, column 2). As the concentration increased to 6.25 μg/ml, germination was slowed uniformly, resulting in an ∼6-h delay, and at 12.5 μg/ml the spore germination was almost completely inhibited, with a very slow increase in circularity observed throughout the population over 16 h (Fig. 3, columns 3 and 4). The presence of a single spore population in all plots indicates uniform inhibition of the spores by pentamidine. That is, there is no subpopulation of spores that is resistant to pentamidine and able to escape inhibition.
FIG 3.
Pentamidine slows germination uniformly across the spore population. Germination profiles of spores (JEC20×JEC21) exposed to 0, 3.13, 6.25, 12.5, 25, or 50 μg/ml pentamidine isethionate in germinating conditions at 0, 4, 8, 12, and 16 h of germination were determined. Each profile assesses ∼10,000 individual cells (top panel). Each germination profile has an aspect ratio going from 0.4 to 1 on the y axis and an area going from 0 to 25 μm2 on the x axis. Quantification of cell populations at each time point and at each concentration is displayed in bar plots (bottom panel).
To characterized the ability of pentamidine to kill a germinating population of Cryptococcus spores we determined its minimum germicidal concentration (MGC) based on complete germicidal activity (killing 100% of cells). Pentamidine demonstrated germicidal activity with an MGC of 50 μg/ml. From these data, we conclude that pentamidine is a germicidal drug that is uniformly effective in the inhibition of Cryptococcus spore germination in vitro.
Pentamidine inhibits both yeast growth and spore germination in vivo.
Because pentamidine was a strong inhibitor of both yeast growth and germination in vitro, we hypothesized that it would inhibit growth and germination in vivo. To determine the antifungal activity of pentamidine in vivo, we used a mouse inhalation model of cryptococcosis to infect mice, treated them with pentamidine, and assessed fungal burdens. Five mice in each group were infected with either 5 × 106 yeast or 2 × 106 spores intranasally. At 24 h postinfection, the mice were treated via intranasal instillation with either 4 mg/kg/day pentamidine isethionate or phosphate-buffered saline (PBS) for three consecutive days. By 24 h postinfection, the spores have germinated into yeast, and the yeast are the established cell type in the lung. On day 4 postinfection, the lungs were collected from all mice, and fungal burdens per gram of tissue were determined (Fig. 4A). Pentamidine-treated mice showed significantly lower fungal burdens in the lung than PBS-treated mice in both yeast-infected (Fig. 4B) and spore-infected (Fig. 4C) mice. Decreased fungal burdens from treatment initiated 24 h after infection indicate that pentamidine is an efficient inhibitor of yeast growth in vivo in established infections.
FIG 4.
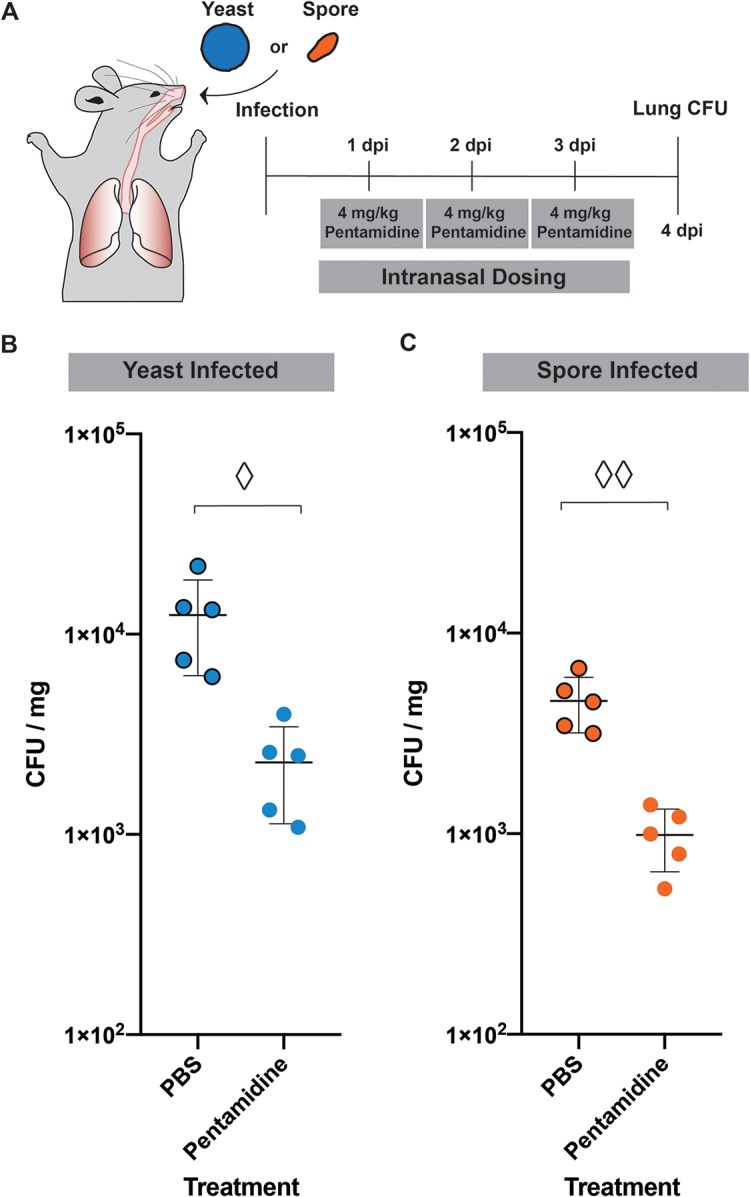
Pentamidine treatment decreases fungal burden in mouse lungs. (A) Timeline of mouse infection and dosing. (B) Lung CFU quantified for each mouse infected with JEC20+JEC21 yeast treated with 4 mg/kg/day pentamidine or with 1× PBS. Diamond, P = 0.0072 (Student t test). (C) Lung CFU quantified for each mouse infected with spores (JEC20×JEC21) treated with 4 mg/kg/day pentamidine or with 1×PBS. Two diamonds, P = 0.0005 (Student t test).
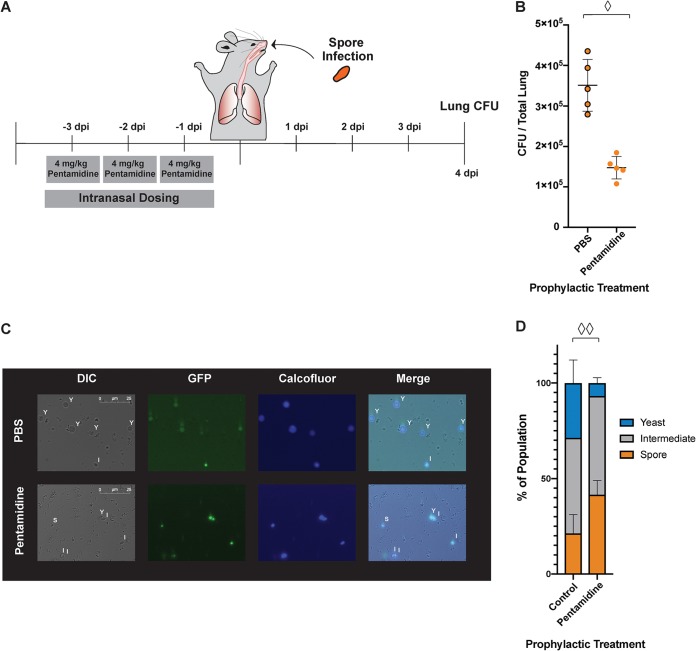
To determine whether or not pentamidine can inhibit germination in vivo, we carried out a modified infection with spores. In this experiment, mice were first treated with pentamidine and then infected with Cryptococcus spores (Fig. 5A). Five mice per group were treated intranasally with either 4 mg/kg/day pentamidine or PBS alone for 3 days. On day 4, all mice were infected with 2 × 106 spores. After a total of 8 days (4 days postinfection), all mice were sacrificed, and lung fungal burdens were determined. We discovered that mice treated prophylactically with pentamidine for 3 days prior to infection had a >2-fold-lower fungal burden than untreated mice (Fig. 5B). This indicates that pentamidine dosing prior to infection can minimize lung fungal burden in spore-mediated infections of mice.
FIG 5.
Prophylaxis with pentamidine inhibits germination in vivo. (A) Timeline of mouse prophylaxis and infection. (B) Lung CFU quantified for each mouse infected with spores (JEC20×JEC21) treated with 4 mg/kg/day pentamidine or with PBS. Diamond, P = 0.0002 (Student t test). (C) Sample images of fixed Cryptococcus cells from bronchoalveolar lavage at 8 h postinfection with spores (CHY3952×CHY3955) with GFP markers and stained with calcofluor white. Y, cells with the properties of yeast; S, cells with the properties of spores; I, intermediates. (D) Quantification of cells retrieved from mouse lungs after prophylaxis with either 1× PBS or pentamidine. Two diamonds, P (yeast) = 0.0370 and P (spores) = 0.0455 (PBS versus pentamidine).
These data were consistent with the inhibition of germination by pentamidine in vivo when administered prior to the initiation of infection. However, we could not rule out that decreases in lung fungal burden were due to inhibition of yeast growth by pentamidine after spore germination. To evaluate the direct effects of pentamidine on spore germination in vivo, we infected mice with spores carrying a green fluorescent protein (GFP) marker, recovered bronchoalveolar lavage fluid from the lungs of prophylactically treated mice 8 h after spore infection, stained the samples with a cell wall dye (calcofluor white), and evaluated the properties of the fungal burden microscopically. Eight hours was chosen for harvest because it was sufficiently long after infection to ensure that spores would have at least initiated the process of germination but early enough to ensure that any yeast present were not the result of vegetative growth. We discovered that cells from pentamidine-treated mice were visibly more consistent with spores in both size and shape than those from untreated mice, which harbored cells more consistent with yeast (Fig. 5C). We quantitated the size and shape of the fungal cells from the lung fluid and detected approximately twice as many spores and 4-fold-fewer yeast in pentamidine-treated mice than in untreated mice (Fig. 5D), indicating that the presence of pentamidine had an inhibitory effect on germination.
These data indicate that treatment with pentamidine prior to infection led to a lung environment in which spore germination was inhibited, ultimately leading to lower fungal burdens. Together, these data demonstrate that prophylactically administered pentamidine has in vivo activity against Cryptococcus spore germination, supporting an argument for further studies of the potential use of pentamidine in the prevention of spore-mediated fungal disease in vulnerable patients.
DISCUSSION
Given the critical need for low-toxicity, high-efficacy antifungal drugs, it is imperative that the field considers new approaches to target human fungal pathogens and spare their hosts. Here, we tested the hypothesis that the process of fungal spore germination could serve as a target for drug-mediated inhibition of differentiation into vegetatively growing cells, ultimately preventing invasive fungal disease. Prior studies of fungal spore germination in other systems led to the suggestion that germination is simply a modified cell cycle in which spores escape dormancy in a process similar to recovery from any static state (e.g., stationary growth phase) (28). However, recent evidence from Cryptococcus suggests that at least some of the events that occur in germination are specific to the process (7). In previous studies we identified and characterized spore-enriched proteins, and one of these proteins (Isp2) appears to play a germination-specific role and no apparent role in normal vegetative growth (29). These Isp2 data and other unpublished observations indicate that germination has properties distinct from a standard cell cycle, suggesting that at least some aspects of germination involve germination-specific proteins. The apparent specificity of the germination process to fungi, as well as the discovery of germination-specific proteins and events, makes germination a promising target for fungus-specific inhibition and drug development.
Currently, there are no approved drugs to prevent infection by Cryptococcus (30, 31). Primary prophylaxis with azoles has been shown to reduce the incidence of cryptococcal disease in adults with advanced HIV; however, there is no evidence of reduction of overall mortality (32). These findings, in combination with other hindrances such as costs and the risks of antifungal drug resistance development, have resulted in azoles not being recommended for routine primary prophylaxis against invasive fungal pathogens (31, 33). In addition to providing a fungus-specific drug target, germination provides a unique opportunity for the prevention of fungal disease. Spores are stress-resistant cell types that are known infectious particles of many fungal pathogens and, in Cryptococcus, spores show distinct disease profiles within mammalian hosts compared to yeast (7, 8, 14). Antifungal agents that target all potential infectious cell types could be used to protect against fungal pathogens through prophylaxis. Such a drug would need to be low toxicity and fungicidal to mitigate against the development of resistance. Prophylactic treatment could be administered to immunocompromised individuals, providing a much-needed option for fungal disease prevention.
The screening of off-patent FDA-approved drugs provides a unique opportunity to screen molecules that often have known molecular targets. Using this approach, we could identify pathways important for fungal spore germination to help understand this critical differentiation process. One of the clearest examples of a potential tool in this study was the drug alexidine, which had strong antifungal activity and was a potent inhibitor of spore germination. This drug has been reported previously to inhibit several phospholipases of Cryptococcus (34). This suggests that phospholipases are important for the germination of fungal spores; however, the direct target of alexidine during germination remains to be determined. In the future, we can use drugs such as alexidine (with known or suspected targets) as tools to probe specific molecular events during germination.
On the other hand, discovering the antifungal properties of drugs could help elucidate their mechanisms of action. For example, a drug with previously unknown antifungal activity that we characterized in this study was otilonium bromide (OB). OB is a quaternary ammonium compound currently approved for use in the treatment of irritable bowel syndrome (IBS) due to its ability to inhibit gastrointestinal motility (35). Recently, OB was determined to have antibacterial activity (36), and in this study we demonstrate the potent antifungal activity of OB against a variety of fungal pathogens. These findings open the possibility that part of the efficacy of OB in IBS could be attributed its antibacterial and/or antifungal activities. In fact, a recent study implicates gut fungi as contributing to morbidity in IBS patients (37). As our understanding of the roles of the gut micro- and mycobiota in human health improves, it could be highly informative to determine the role of the antimicrobial activity of OB in the treatment of IBS.
Screening FDA-approved drugs also has the benefit of providing drugs that could reach patients in need sooner than novel compounds. In this study, we found that the antiparasitic drug pentamidine shows particular promise for repurposing as a germination inhibitor for numerous reasons. First, pentamidine is only approved for use against a single fungus, Pneumocystis (formerly classified as a protozoan parasite), which causes pneumonia, largely in people with AIDS (24, 25). Second, pentamidine is approved for use in immunocompromised individuals, which is the primary group affected by cryptococcosis (24, 38). Third, pentamidine already exists in an aerosolized formulation, which allows for noninvasive administration (26). Fourth, the drug is known to accumulate in the lungs, which is the main site where Cryptococcus establishes infections (27). Finally, this drug is already approved for use prophylactically, which would facilitate its use in at-risk patients (24, 26, 38).
Our findings that pentamidine was effective at lowering the fungal burden in a mouse model of Cryptococcus infection and was able to inhibit spore germination in vivo indicate that pentamidine can accumulate in and remain in the lungs long enough to exert its antifungal and antigerminant effects. Because pentamidine can be quite toxic to humans when administered intravenously, it is often not the first choice for prophylaxis against Pneumocystis in humans (39, 40). However, when pentamidine is administered as an aerosol in the lung, there are fewer issues with toxicity (26). Our study suggests that pentamidine could be used as a prophylactic drug that could protect patients against Cryptococcus infections and thus warrants further clinical investigation. Finally, these data indicate that fungal spore germination can be an effective target in the development of novel antifungal strategies.
In this study, we demonstrate that spore germination is a viable target for antifungal drug development by identifying and characterizing FDA-approved drugs that exhibit antigerminant activity. These germination inhibitors have the potential to be repurposed into new antifungal therapies. In particular, robust, low-toxicity antigerminants could be used prophylactically in at-risk patients to prevent the development of fulminant spore-mediated diseases. Supporting the feasibility of this idea, we determined that one of the drugs we identified, pentamidine, was effective at lowering the fungal burden in vivo by inhibiting both germination and subsequent yeast growth. Pentamidine was effectively used as a prophylactic treatment against cryptococcal infection in mice and serves as an exemplar of the potential for use of FDA-approved drugs in spore-mediated fungal disease prevention.
MATERIALS AND METHODS
Strains and strain manipulation.
The following strains were used and handled using standard techniques and media as previously described (41, 42): Cryptococcus neoformans serotype D, strains JEC20, JEC21, CHY3952 (JEC20+GFP), and CHY3955 (JEC21+GFP) (14), and serotype A, strain H99; Candida albicans strain SC5314; and Aspergillus fumigatus strain AF293. Cryptococcus spores were isolated from cultures as previously described (43). Briefly, yeast of both mating types (JEC20 and JEC21) were grown on yeast-peptone-dextrose (YPD) medium for 2 days at 30°C combined in PBS, mixed to a 1:1 ratio, and spotted onto V8 pH 7 agar plates. Plates were incubated for 5 days at 25°C, and spots were resuspended in 70% Percoll in 1× PBS and subjected to gradient centrifugation. Spores were recovered and counted using a hemacytometer. Aspergillus strains were maintained as glycerol stocks at –80°C and propagated on glucose-containing minimal medium at 37°C (41). Conidia were collected using 0.01% Tween 80 in PBS after 3 days of growth on glucose medium plates, counted using a hemacytometer, and used in MIC studies immediately postharvest.
High throughput screen of drug library.
All screening of the L1300 Selleck Library (purchased from Selleck Chemicals) was carried out with the assistance of the University of Wisconsin—Madison (UW-Madison) Small Molecule Screening Facility. To identify spore germination inhibitors, we generated a reporter strain harboring a protein-luciferase fusion construct. The CNK01510 protein product was identified in a previous proteomic study and was therefore known to be present in spores (29). The product of CNK01510 (Isp4) has no similarity to any known proteins. Open reading frame CNK01510 from Cryptococcus strain JEC21 was fused to nanoluciferase (NL; Promega Corporation) using overlap PCR. The resulting fragment, which contained flanking sequences for homologous integration into the CNK01510 locus, was transformed into JEC20 and JEC21. Multiple independent transformants in both mating types were verified using PCR, Southern blotting, and NL activity for proper construct integration into the desired locus (and only that locus) and proper NL expression. All strains exhibited the same behaviors, and strains CHY3833 and CHY3836 were then chosen for producing spores for library screening. Screening was carried out with 1 × 104 spores incubated in 384-well plates in 10 μl of germination medium (0.5× YPD) with or without drugs for 10 h at 30°C, at which point the cell density was measured based on the OD600. Cells were then incubated with 10 μl of Nano-Glo luciferase assay reagent (Promega Corporation) prepared as suggested by the manufacturer at 22°C for 10 min and then read using a Perkin-Elmer Enspire plate reader. All screening resulted in Z′ values of 0.7 or better for both NL and OD600 readings. The Selleck Library was screened in duplicate independently three times, and all values shown are representative of each screen. Additional details of the screen will be published in a subsequent manuscript.
MIC/MFC assays.
All MIC experiments with yeast were based on CLSI methodology (19). MIC values are defined as the lowest concentration giving rise to an inhibition of growth ≥90% of the drug-free control. Briefly, log-phase yeast cells were washed in 1× PBS and quantified using a hemacytometer. For each drug, 1.25 × 105 yeast were incubated in RPMI and 0.33 M morpholinepropanesulfonic acid (MOPS; pH 7) with various concentrations of inhibitors, with a final volume of 200 μl. Cryptococcus yeast were incubated for 2 days at 30°C, whereas Candida albicans strains were incubated for 2 days at 35°C. OD600 readings were used to assess the MIC values for each drug. For this study, we defined MFC as the lowest concentration at which no fungal growth was observed after 2 days of exposure to drug (post-MIC90 treatment) and 2 additional days of growth in the absence of drug. MFCs were determined by plating 5% of the suspension used to determine the MIC onto YPD plates. The plates were visually assessed after 48 h, and the concentration at which no CFU were observed (for every replicate) was determined to be the MFC.
For Aspergillus fumigatus MICs, we used the EUCAST methodology (20). Briefly, conidia at a final concentration of 105 conidia/ml were incubated in RPMI, 0.33 M MOPS, and 2% glucose at pH 7 with various concentrations of inhibitors, with a final volume of 200 μl. The MIC values were then assessed based on the lowest concentration of drug that showed a complete absence of germ tubes or hyphae.
Quantitative germination assay.
All germination assays are based on Barkal et al. (15). Microfluidic devices were loaded with 105 spores, and at 0 h YPD medium containing the drug of interest was added to the sample. Primary testing was performed at 25 μg/ml for all drugs to ensure that at least an ∼5-fold concentration of the MIC was tested because spores are more stress resistant than yeast and therefore a higher concentration is required to observe germination inhibition. Spores were allowed to germinate at 30°C in a humidified chamber, and cells were monitored every 2 h for 16 h. Each assay was performed in two individual wells with three fields of view acquired from each well. All images were analyzed as previously described based on cell shape and size. The population ratios of spores, intermediates, and yeast were determined. Error bars in plots are based on the variation between all fields of view acquired. All experiments were reproduced independently. After 16 h, samples were plated on YPD and allowed to grow at 30°C to determine whether drugs were fully germicidal based on lack of growth. If assays could not be performed in microfluidic devices due to the chemical properties of a given drug, then 2 × 105 spores were incubated in identical conditions and loaded into devices only for image acquisition.
Minimum germicidal concentrations (MGCs) were determined by plating spores onto YPD after 16 h of incubation at each drug concentration during the germination assay, and the plates were incubated at 30°C. After 3 days, the plates were scored for the presence or absence of growth. MGC values were determined as the lowest concentration at which no colonies grew on the plates, indicating complete germicidal activity (killing of 100% of the cells).
In vivo efficacy assessment assay.
All experiments were performed on 8- to 10-week-old C57BL/6J (Jackson Laboratory) female mice (five mice per group). All mice were infected intranasally with either yeast or spores with a total volume of 50 μl. All yeast were cultured overnight in YPD, washed, and used to infect each mouse with a total of 5 × 106 yeast comprising of a 1:1 ratio of JEC20 and JEC21 strains. Spores (JEC20×JEC21) were isolated as described above, and each mouse was infected with 2 × 106 spores. All drug dosing was performed with either 4 mg/kg/day pentamidine isethionate or 1× PBS for 3 days, either prior to infection or 1 day postinfection. Mice were sacrificed 4 days postinfection, and their lungs were collected, processed, and evaluated for fungal burdens. All pairwise comparisons were evaluated using a two-tailed unpaired Student t test.
In vivo germination assessment assay.
Female mice, 8 to 10 weeks old C57BL/6J (Jackson Laboratory; three mice per group), were dosed with either 4 mg/kg/day pentamidine isethionate or 1× PBS (50 μl) for 3 consecutive days. Mice were infected intranasally with 2 × 106 spores derived from crosses between CHY3952 and CHY3955. At 8 h postinfection, the mice were sacrificed, and their lungs were lavaged with 0.05% Triton-X in 1× PBS to recover Cryptococcus cells. The 8-h time point was selected because pilot experiments showed that 4 h was too early to distinguish ungerminated spores from germinating spores and that 12 h was too late to distinguish yeast resulting directly from spore germination from subsequently replicating yeast. The resulting bronchoalveolar lavage suspension was treated, in order: red blood cell lysis (ACK lysing buffer, 2 ml, 5 min), formaldehyde fixation (4%, 500 μl, 30 min), and calcofluor white staining (25 μg/ml, 20 μl, 1 min). We imaged 50 to 100 cells per mouse that were identified as Cryptococcus based on green fluorescent signal or cyan signal from calcofluor staining. Cell surface areas and aspect ratios were measured in ImageJ, and cells were classified as spores, intermediates, or yeast. All pairwise comparisons were evaluated using a two-tailed unpaired Student t test.
Ethics statement.
All experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health under University of Wisconsin—Madison (UW-Madison) Institutional Animal Care and Use Committee-approved protocol number A005118. UW-Madison is an AAALAC-accredited institution.
Supplementary Material
ACKNOWLEDGMENTS
We thank the N. Keller lab at UW-Madison for sharing Aspergillus strains. We thank James (Muse) Davis for his microscopy brilliance in helping to automate our germination assay. We also thank the University of Wisconsin Optical Imaging Core for the use of their microscopes and the staff at the Small Molecule Screening Facility at the University of Wisconsin Cancer Carbone Center for their assistance and expertise with support from National Institutes of Health (NIH) grant P30 CA014520. Finally, we thank the animal care staff in the UW-Madison BABS and MSB facilities for their consistent and high-quality animal care.
This study was supported by an Individual Biomedical Research Award from The Hartwell Foundation to C.M.H., an NIH grant R01 AI137409 to C.M.H., and an HHMI Gilliam Fellowship to S.C.O.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00994-19.
REFERENCES
- 1.Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases: estimate precision. J Fungi 3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roemer T, Krysan D. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. 2018. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 742:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 2017. Stop neglecting fungi. Nat Microbiol 2:17120. doi: 10.1038/nmicrobiol.2017.120. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:1–10. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 6.Odds FC, Brown AJP, Gow N. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 7.Huang M, Hull CM. 2017. Sporulation: how to survive on planet Earth (and beyond). Curr Genet 63:831–838. doi: 10.1007/s00294-017-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sephton-Clark PCS, Voelz K. 2018. Spore germination of pathogenic filamentous fungi. Adv Appl Microbiol 102:117–157. doi: 10.1016/bs.aambs.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Oprea TI, Mestres J. 2012. Drug repurposing: far beyond new targets for old drugs. AAPS J 14:759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin X, Heitman J. 2006. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 11.Perfect JR, Casadevall A. 2002. Cryptococcosis. Infect Dis Clin North Am 16:837–874. doi: 10.1016/S0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 12.Giles SS, Dagenais TRT, Botts MR, Keller NP, Hull CM. 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun 77:3491–3500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velagapudi R, Hsueh Y-P, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 77:4345–4355. doi: 10.1128/IAI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh NM, Botts MR, McDermott AJ, Ortiz SC, Wüthrich M, Klein BS, Hull CM. 2019. Infectious particle identity determines dissemination and disease outcome for the inhaled human fungal pathogen Cryptococcus. PLoS Pathog 15:e1007777. doi: 10.1371/journal.ppat.1007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkal LJ, Walsh NM, Botts MR, Beebe DJ, Hull CM. 2016. Leveraging a high resolution microfluidic assay reveals insights into pathogenic fungal spore germination. Integr Biol 8:603–615. doi: 10.1039/c6ib00012f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemecek JC, Wüthrich M, Klein BS. 2006. Global control of dimorphism and virulence in fungi. Science 312:583–1202. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhary A, Randhawa HS, Sundar G, Kathuria S, Prakash A, Khan Z, Sun S, Xu J. 2011. In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from northwestern India. J Med Microbiol 60:961–967. doi: 10.1099/jmm.0.029025-0. [DOI] [PubMed] [Google Scholar]
- 18.Altshuler B. 1981. Modeling of dose–response relationships. Environ Heal Perspect 42:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2008. Approved standard M27-A3: reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ, the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2015. EUCAST definitive document E.DEF 9.3: method for the determination of broth dilution minimum inhibitory concentration for conidia forming moulds. European Committee for Antimicrobial Susceptibility Testing, Copenhagen, Denmark. [Google Scholar]
- 21.Barchiesi F, Del Poeta M, Morbiducci V, Ancarani F, Scalise G. 1994. Effect of pentamidine on the growth of Cryptococcus neoformans. J Antimicrob Chemother 33:1229–1232. doi: 10.1093/jac/33.6.1229. [DOI] [PubMed] [Google Scholar]
- 22.Lackner TE, Clissold SP. 1989. Bifonazole: a review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs 38:204–225. doi: 10.2165/00003495-198938020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Heel RC, Brogden RN, Speight TM, Avery GS. 1978. Econazole: a review of its antifungal activity and therapeutic efficacy. Drugs 16:177–201. doi: 10.2165/00003495-197816030-00001. [DOI] [PubMed] [Google Scholar]
- 24.Magill AJ, Hill DR, Solomon T, Ryan ET (ed). 2012. Hunter’s tropical medicine and emerging infectious disease, 9th ed W.B. Saunders, Philadelphia, PA. [Google Scholar]
- 25.Stringer JR. 1993. The identity of Pneumocystis carinii: not a single protozoan, but a diverse group of exotic fungi. Infect Agents Dis 2:109–117. [PubMed] [Google Scholar]
- 26.Leoung GS, Feioal DW, Montgomery AB, Corkery K, Wardlaw L, Adams M, Busch D, Gordon S, Jacobson MA, Volberding PA, Aurams D, The San Francisco County Community Consortium. 1990. Aerosolized pentamidine for prophylaxis against Pneumocystis carinii pneumonia. N Engl J Med 323:769–775. doi: 10.1056/NEJM199009203231201. [DOI] [PubMed] [Google Scholar]
- 27.Stockmann C, Roberts JK, Yellepeddi VK, Sherwin C. 2015. Clinical pharmacokinetics of inhaled antimicrobials. Clin Pharmacokinet 54:473–492. doi: 10.1007/s40262-015-0250-x. [DOI] [PubMed] [Google Scholar]
- 28.Joseph-Strauss D, Zenvirth D, Simchen G, Barkai N. 2007. Spore germination in Saccharomyces cerevisiae: global gene expression patterns and cell cycle landmarks. Genome Biol 8:R241–R245. doi: 10.1186/gb-2007-8-11-r241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang M, Hebert AS, Coon JJ, Hull CM. 2015. Protein composition of infectious spores reveals novel sexual development and germination factors in Cryptococcus. PLoS Genet 11:e1005490. doi: 10.1371/journal.pgen.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, Havens P, Nesheim S, Read JS, Serchuck L, Van Dyke R. 2009. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children. MMWR Recomm Rep 58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. 2002. Guidelines for preventing opportunistic infections among HIV-infected persons—2002 recommendations of the U.S. public health service and the infectious diseases society of America. MMWR Morb Mortal Wkly Rep 51:1–52. [PubMed] [Google Scholar]
- 32.Powderly WG, Finkelstein DM, Feinberg J, Frame P, He W, Van der Horst C, Koletar SL, Eyster ME, Carey J, Waskin H, Hooton TM, Hyslop N, Spector SA, Bozzette SA. 1995. The NIAID AIDS Clinical Trials Group. 1995. A randomized trial comparing fluconazole with clotrimazole troches for prevention of fungal infection in patients with advanced human immunodeficiency virus infection. N Engl J Med 332:700–705. doi: 10.1056/NEJM199503163321102. [DOI] [PubMed] [Google Scholar]
- 33.Chang LW, Phipps WT, Kennedy GE, Rutherford G. 2005. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database Syst Rev 3:CD004773. doi: 10.1002/14651858.CD004773.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Ganendren R, Widmer F, Singhal V, Wilson C, Sorrell T, Wright L. 2004. In vitro antifungal activities of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob Agents Chemother 48:1561–1569. doi: 10.1128/aac.48.5.1561-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evangelista S. 1999. Otilonium bromide: a selective spasmolytic for the gastrointestinal tract. J Int Med Res 27:207–222. doi: 10.1177/030006059902700501. [DOI] [PubMed] [Google Scholar]
- 36.Knauf GA, Cunningham AL, Kazi MI, Riddington IM, Crofts AA, Cattoir V, Trent MS, Davies BW. 2018. Exploring the antimicrobial action of quaternary amines against Acinetobacter baumannii. mBio 9:1–13. doi: 10.1128/mBio.02394-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, de Weerd HH, Boekhout T, Fornai M, Masclee AA, Schuren FHJ, de Jonge WJ, Seppen J, van den Wijngaard RM. 2017. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 153:1026–1039. doi: 10.1053/j.gastro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. 2019. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 13 May 2019.
- 39.Balslev U, Nielsen TL. 1992. Adverse effects associated with intravenous pentamidine isethionate treatment of Penumocystis carinii pneumonia in AIDS patients. Dan Med Bull 39:366–368. [PubMed] [Google Scholar]
- 40.Schneider MME, Hoepelman AIM, Schattenkerk JKME, Nielsen TL, van der Graaf Y, Frissen JPHJ, van der Ende IME, Kolsters AFP, Borleffs JCC, The Dutch AIDS Treatment Group. 1992. A controlled trial of aerosolized pentamidine or trimethoprim–sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. N Engl J Med 327:1836–1841. doi: 10.1056/NEJM199212243272603. [DOI] [PubMed] [Google Scholar]
- 41.Sherman F, Fink GR, Hicks JB. 1987. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 42.Shimizu K, Keller NP. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botts MR, Giles SS, Gates MA, Kozel TR, Hull CM. 2009. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot Cell 8:595–605. doi: 10.1128/EC.00352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.