Human T-lymphotropic viruses type 1 and 2 (HTLV-1/2) are prevalent in endemic clusters globally, and HTLV-1 infects at least 5 to 10 million individuals. Infection can lead to inflammation in the spinal cord, resulting in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), or adult T cell leukemia/lymphoma (ATL).
KEYWORDS: ELISA, HTLV-1, HTLV-2, IgG, diagnostics, gingival crevicular fluid, human T-cell leukemia virus, immunoassays, immunodiagnostics, oral fluid
ABSTRACT
Human T-lymphotropic viruses type 1 and 2 (HTLV-1/2) are prevalent in endemic clusters globally, and HTLV-1 infects at least 5 to 10 million individuals. Infection can lead to inflammation in the spinal cord, resulting in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), or adult T cell leukemia/lymphoma (ATL). Obtaining venous blood for serological screening, typically performed using enzyme immunoassays (EIAs), is invasive, sometimes socially unacceptable, and has restricted large-scale seroprevalence studies. Collecting oral fluid (OF) is a noninvasive alternative to venesection. In this study, an IgG antibody capture EIA was developed and validated to detect anti-HTLV-1/2 IgG in OF. OF and plasma specimens were obtained from seropositive HTLV-1/2-infected patients attending the National Centre for Human Retrovirology (n = 131) and from HTLV-1/2-uninfected individuals (n = 64). The assay showed good reproducibility and high diagnostic sensitivity (100%) and specificity (100%) using both OF and plasma. The Murex HTLV I+II commercial assay was evaluated and did not detect anti-HTLV-1/2 IgG in 14% (5/36) of OF specimens from seropositive donors. The reactivities of OF and plasma in the IgG capture correlated strongly (r = 0.9290) and were not significantly affected by delayed extraction when held between 3°C and 45°C for up to 7 days to simulate field testing. The use of OF serological screening for HTLV-1/2 infection could facilitate large-scale seroprevalence studies, enabling active surveillance of infection on a population level.
INTRODUCTION
Human T-lymphotropic viruses type 1 and 2 (HTLV-1/2) are related retroviruses of the Deltaretrovirus genus. HTLV-1 infects at least 5 to 10 million people globally in endemic clusters which include regions in southwestern Japan, Australo-Melanesia, the Caribbean, South America, sub-Saharan Africa, and the Middle East (1). Infection is mostly acquired through sexual transmission or mother-to-child transmission (primarily prolonged breastfeeding) (2). Infection is lifelong and is a cause of significant morbidity and mortality. Those infected may develop HTLV-1-associated inflammation, of which HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is the best known, or malignancy, namely, adult T-cell leukemia/lymphoma (ATL) (3, 4). HTLV-1 infection also impacts a number of coinfections, including human immunodeficiency virus (HIV) infection, strongyloidiasis, Norwegian scabies, and tuberculosis (5–8). The clinical consequences of HTLV-2 infection are less well understood, but an atypical form of myelopathy has been reported, along with increased urinary and respiratory infections (9, 10).
Serological assays are the principle method for the diagnosis of infection, including in prevalence studies, and HTLV-associated diseases (1, 11, 12). Enzyme immunoassays (EIAs) are most commonly employed for initial screening due to their ease of use and low cost. The performance of different EIAs can vary depending on assay format, analyte, and viral antigens used (13). Western blot analyses are frequently performed for confirmation of screening assay reactivity and to differentiate between HTLV-1 and HTLV-2.
Since acquiring peripheral venous blood is invasive and unacceptable in some populations, performing serum- or plasma-based serological tests can be difficult, especially among pediatric and certain geographically isolated Indigenous populations. Collecting blood from Indigenous populations has historically been hindered by cultural and religious barriers, while securing venous access in children is challenging (14, 15). High rates of infection have been reported in both populations in regions where HTLV is endemic, but the number of children tested is often limited (16–18). Current treatments for HTLV-associated diseases are unsatisfactory, and no vaccine exists; thus, prevention of infection is key. Improving screening coverage is essential for surveillance, accurately mapping disease occurrence, and targeting resources for infection control, including prevention of maternal-infant transmission.
Various noninvasive sources of bodily fluids for serology have been evaluated. Oral fluid (OF) includes a plasma transudate known as gingival crevicular fluid (GCF). GCF accumulates in the crevice between the tooth and the gum and contains IgG at an 800- to 1,000-fold lower concentration than that of serum (19). Obtaining GCF-rich OF has previously been shown to be rapid, safe, and well tolerated (15, 20, 21), and it provides an analyte used globally for the monitoring and diagnosis of measles virus infection. According to Vyse et al., most parents found collecting OF from 3.5- to 5-year-old children to be easily performed, and few objected to repeating the process (15).
Although anti-HTLV-1/2 antibodies and proviral DNA have been detected in OF, there has been limited exploration of the clinical and epidemiological applicability of this approach (22–25). The aim of this study was to develop and validate an IgG antibody capture EIA to detect anti-HTLV-1/2 IgG in GCF-rich OF suitable for surveillance of HTLV-1/2 infection at a population level.
MATERIALS AND METHODS
Ethical considerations.
Written informed consent was obtained from all named individuals prior to sample collection. The approval of use of UK samples for HTLV research was delegated to the Communicable Diseases Research Tissue Bank by the National Research Ethics Service (NRES) South Central Oxford C Research Ethics Committee (approval number 15/SC/0089). Residual anonymized OF samples taken for outbreak control purposes were available for EIA validation.
Clinical samples.
One hundred Western blot-confirmed (HTLV 2.4; Genelabs Diagnostics, Singapore) HTLV-1/2-seropositive patients attending the National Centre for Human Retrovirology, St Mary’s Hospital, London, were enrolled between January and May 2019, donating 131 OF and plasma paired samples. Their median age was 58 (range, 25 to 81) years. A single patient was infected with HTLV-2. Nine HTLV-1-infected patients were coinfected with HIV, two of whom had HAM/TSP. Of the 90 remaining HTLV-1 infected individuals, 34 had HAM/TSP, 10 had ATL, 35 were asymptomatic carriers, two had arthritis, three had presented with Strongyloides stercoralis infection, and six manifested neurological disorders not attributed to HAM/TSP.
Paired OF and plasma samples were donated by 13 adult volunteers with no history of HTLV-1/2 infection and OF only was donated by an HIV monoinfected individual. In addition, a panel of 50 anonymized and randomly selected OF specimens from presumed HTLV-1/2-seronegative individuals (low HTLV-1/2 risk) from the United Kingdom were donated by Public Health England. These specimens were previously validated for a total IgG quantity of >1 mg/liter.
Sample collection.
Peripheral venous blood was collected into EDTA-containing tubes, and plasma was separated by density centrifugation and then stored at −80°C until assayed. GCF-rich OF was collected using the Oracol device (Malvern Medical Developments, Worcester, UK) according to the manufacturer’s instructions. The swab was processed by adding 1 ml transport medium (10% fetal calf serum, 0.5% gentamicin, and 0.2% amphotericin B in phosphate-buffered saline [PBS]), followed by manual agitation. The fluid was recovered from the sponge using a twisting motion. After centrifugation (2,000 rpm for 5 min), the eluate was transferred into labeled vials and stored at −80°C. The majority of OF samples were processed the same day (mean time from OF collection to storage of 3 h and 26 min (range, 30 min to 7 h and 43 min); however, 12 samples arrived too late for same-day processing. One was held at room temperature, five at 3°C, two at −20°C, and four at −80°C for up to 7 days before extraction.
Enzyme immunoassays.
An EIA based on an IgG capture format was developed. Microwells were coated with 100 μl of 5 μg/ml rabbit anti-human IgG (Stratech Scientific, Ely, UK) in coating buffer (Clintech) and incubated overnight at 2 to 8°C. Wells were then washed with PBS/0.05% Tween 20 once and blocked using 200 μl/well blocking solution (Microimmune, Guildford, UK) before drying overnight at 37°C. For testing plasma, samples were diluted 1:200 in transport medium containing an addition of 0.1% Tween 20, 100 μl of which was then added to the wells. For OF testing, 100 μl was added directly to each well. The wells were then incubated for 1 h at 37°C. Wash fluid was prepared with 10% wash buffer (Microimmune) in distilled water. The wells were washed five times, and 25 μl horseradish peroxidase-labeled conjugate comprising HTLV-1 recombinant transmembrane protein and HTLV-1 and HTLV-2 envelope-based synthetic peptides from the Murex HTLV I+II EIA (DiaSorin, Dartford, UK) and 25 μl/well of conjugate diluent (Clin-Tech, Guildford, UK) were added at the same time to the well. After incubation for 2 h at 37°C, the plate was washed five times and 100 μl/well TMB substrate (Clin-Tech) was added, followed by a further 30-min incubation at 37°C. Reactions were stopped using 50 μl/well 0.5 M sulfuric acid (Microimmune). Raw optical densities (ODs) were determined by the SpectraMax M2 microplate analyzer (Molecular Devices, San Jose, CA, USA) at 450 nm. A cutoff value was calculated from the mean optical density (OD) of the negative-control triplicate on a plate plus 0.1. To normalize ODs between plates, a signal-to-cutoff ratio (S/CO) was calculated for each sample by dividing its raw OD by the cutoff value. A sample was considered reactive if it gave a S/CO of ≥1.00.
Paired OF and plasma samples from 36 HTLV-1-infected patients and 8 uninfected individuals were randomly selected and also tested in the commercially available Murex HTLV I+II EIA (DiaSorin, Dartford, UK). The assay was performed according to the manufacturer’s instructions.
Reproducibility.
The reproducibility of the IgG capture assay was assessed by determining intra- and interassay coefficients of variance (CV%). Intra-assay variability was evaluated by testing one HTLV-1 and one HTLV-2 plasma sample eight times each in the same assay. For interassay variability, seven different HTLV-1 samples and one HTLV-2 plasma sample were tested in duplicate on three separate days. The S/CO values of each duplicate were averaged and the mean and standard deviation over three plates determined. To assess the reproducibility of oral fluid samples, two pools were tested. One pool consisted of 15 OF samples with low OD values (<1), while the other pool consisted of 15 samples with high OD values (>3), previously determined using the IgG capture assay. The intra- and interassay coefficients of variance were determined as for plasma.
Assay performance.
Diagnostic sensitivity of the IgG capture assay was evaluated for all 131 OF and plasma samples from the 100 HTLV-1/2-seropositive patients. Sensitivity was calculated as the percentage of known seropositive samples that were reactive in the assay, and specificity as the percentage of 63 OF and 13 plasma samples from 63 HTLV-1/2-uninfected individuals that was nonreactive. Ninety-five percent confidence intervals (95% CI) were determined.
Analytical robustness of the IgG capture assay was evaluated by performing 2-fold serial dilutions of both plasma and oral fluid from two HTLV-1-seropositive donors and one HTLV-2-seropositive donor in transport medium until the samples became nonreactive. In order to confirm the characteristic of an IgG capture assay to be a proportionality assay, plasma from one HTLV-1- and one HTLV-2-infected individual were similarly diluted in plasma pooled from seronegative donors. S/CO values were then plotted against dilution factors.
To test the reproducibility of anti-HTLV-1 antibody detection over time, paired OF and plasma samples were obtained from 23 HTLV-1-infected individuals, with a range of clinical status, at two time points a mean of 3.3 months apart (minimum, 1 month; maximum, 7 months).
Impact of preanalytical conditions on antibody measurement.
The effect of storage temperature on the detection of anti-HTLV-1/2 IgG was investigated. In total, 32 pairs of OF samples were obtained simultaneously from HTLV-1-seropositive patients. In every pair, one sample was processed on the day of collection, while the other was first placed either at 3°C (fridge), room temperature, 37°C (incubator), or 45°C (water bath) for 24 h (5 pairs for each condition). In addition, 6 pairs each were used to examine two critical conditions (3°C and 45°C) for 7 days. Paired samples were assayed and S/CO values compared. Additionally, the effect of freezing and thawing on sample reactivity was examined. Two processed HTLV-1 antibody-containing OF specimens in storage were selected (one with a high and one with a low S/CO value) and each divided into aliquots. Aliquots then completed 5 or 10 successive freeze-thaw cycles of at least 1 h frozen and 2 h thawed. S/CO values were compared to reactivity obtained after one freeze-thaw cycle only.
Statistical analysis.
Data were analyzed using Prism 8 software (GraphPad, San Diego, CA, USA).
Spearman’s two-tailed rank correlation analysis was performed to compare S/CO values between paired HTLV-1/2 antibody-containing OF and plasma samples. Where paired samples were analyzed, the Wilcoxon signed-rank test was conducted to compare differences in S/CO values. S/CO values of infected patients with different clinical phenotypes were compared using the Mann-Whitney U test. Results were considered statistically significant if the P value was <0.05.
RESULTS
Reproducibility.
The reproducibility of the IgG capture assay within and between runs was assessed (Table 1). Intra-assay coefficients of variance for the reactivity of HTLV-1 and HTLV-2 plasma samples, expressed as S/CO ratios, were 3.1% and 2.9%, respectively. The interassay variability ranged between 4.4% and 10.8% for HTLV-1 samples and was 1.1% for the HTLV-2 sample. The intra-assay variability for the pooled OF samples with low and high OD values was 2.6% and 4%, respectively. The interassay variability for these pooled samples was 8.7% and 6.7%, respectively.
TABLE 1.
Reproducibility of the IgG capture assay
| Sample | Mean S/CO (SD)a | CV%b |
|---|---|---|
| Intra-assay variability | ||
| Plasma | ||
| HTLV-1 sample A | 21.84 (0.69) | 3.1% |
| HTLV-2 sample B | 3.05 (0.09) | 2.9% |
| Oral fluid | ||
| Pool with low S/CO | 5.55 (0.15) | 2.65% |
| Pool with high S/CO | 20.91 (0.84) | 4% |
| Interassay variability | ||
| Plasma | ||
| HTLV-1 sample A | 21.63 (1.39) | 6.4% |
| HTLV-1 sample B | 7.25 (0.64) | 8.9% |
| HTLV-1 sample C | 21.27 (0.93) | 4.4% |
| HTLV-1 sample D | 19.73 (1.17) | 5.9% |
| HTLV-1 sample E | 8.73 (0.94) | 10.8% |
| HTLV-1 sample F | 6.39 (0.33) | 5.2% |
| HTLV-1 sample G | 5.35 (0.35) | 6.5% |
| HTLV-2 sample A | 2.96 (0.03) | 1.1% |
| Oral fluid | ||
| Pool with low S/CO | 6.05 (0.53) | 8.7% |
| Pool with high S/CO | 22.36 (1.5) | 6.7% |
S/CO, signal-to-cutoff ratio; SD, standard deviation.
CV%, coefficient of variation.
Assay performance.
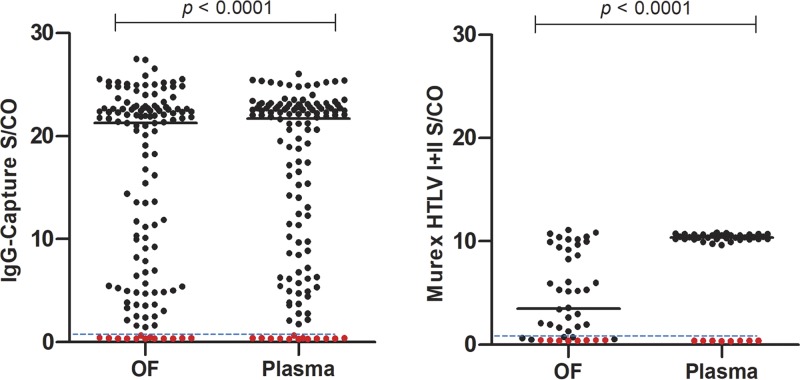
All 131 paired OF and plasma samples from Western blot-confirmed HTLV-1/2-seropositive patients were reactive in the IgG capture assay, giving a sensitivity of 100% (95% CI, 97.2% to 100%) for both OF and plasma. Based on the 100 patients’ first time point sample (i.e., excluding the repeated samples), the 95% CI is 96.4% to 100%. All 13 OF and plasma pairs from uninfected adult volunteers were nonreactive, as were all 50 unpaired OF specimens from presumed seronegative individuals. The OF specimen from an HIV monoinfected patient was also nonreactive, thereby giving an overall specificity for both OF and plasma of 100% (95% CI, 94.4% to 100% and 75.3% to 100%, respectively) (Fig. 1).
FIG 1.
Comparison of paired OF and plasma samples, expressed as S/CO values. (Left) One hundred seropositive (n = 131 paired samples) and 13 seronegative individuals in the IgG-capture assay. (Right) Thirty-six seropositive and 8 seronegative individuals in the Murex HTLV I+II EIA. Black dots, samples from seropositive individuals; red dots, samples from seronegative individuals. Solid horizontal bars show median. The horizontal dashed line indicates the S/CO at 1.00, above which samples are considered reactive.
In total, 36 paired OF and plasma samples from seropositive patients were assayed in the Murex HTLV I+II EIA for comparison, following the manufacturer’s instructions for serum. While all 36 plasma samples were reactive, 5 of 36 OF samples were nonreactive, giving sensitivities of 100% (95% CI, 90.3% to 100%) and 86% (95% Cl, 70.5% to 95.3%), respectively. The OF samples that were nonreactive in the Murex HTLV I+II EIA all had low reactivities in the IgG capture assay, with S/CO values that ranged between 1.46 and 11.39. All eight paired OF and plasma specimens from seronegative individuals were nonreactive, yielding a specificity of 100% (95% CI, 63.1% to 100%) (Fig. 1).
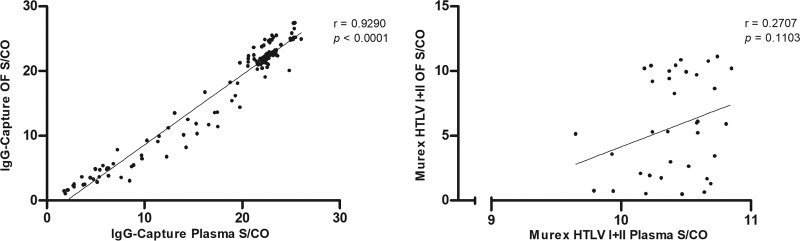
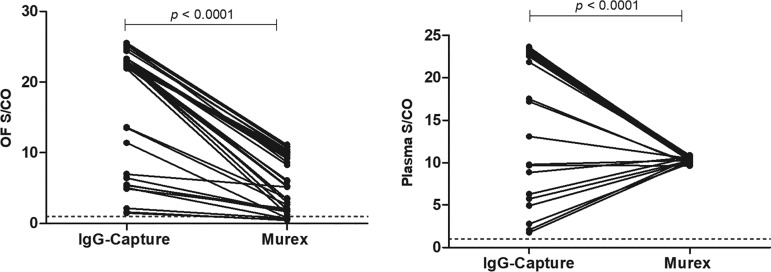
The correlation of S/CO values of paired OF and plasma samples from infected patients was strong (Spearman’s rank correlation coefficient [rs] = 0.9290; P < 0.0001) with the IgG capture assay but not with the Murex HTLV I+II EIA (rs = 0.2707; P = 0.1103) (Fig. 2). A range of S/CO values is seen with the IgG capture assay and the Murex HTLV I+II EIA for OF but only with the IgG capture assay for plasma (Fig. 1 and 3). The differences between medians of paired OF and plasma reactivities were 3.33 (P < 0.0001) and 0.7731 (P < 0.0001) with the Murex HTLV I+II EIA and the IgG capture assay, respectively (Fig. 1).
FIG 2.
Correlation of 131 paired OF and plasma samples from 100 seropositive individuals, expressed as S/CO values, in the IgG-capture assay (left), and 36 paired OF and plasma samples from seropositive individuals in the Murex HTLV I+II EIA (right). Spearman’s rank correlation coefficient (rs) is shown. P value indicates degree of statistical significance.
FIG 3.
Comparison of OF (left) and plasma (right) samples analyzed by IgG-capture assay and Murex HTLV I+II EIA. The horizontal dashed line indicates the S/CO at 1.00, above which samples are considered reactive.
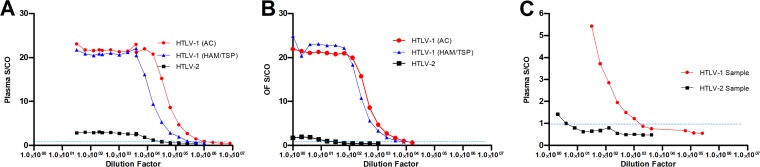
The analytical robustness was evaluated to determine the range of concentrations over which anti-HTLV-1/2 antibodies in samples remained detectable in the IgG capture (Fig. 4). When diluted in transport medium, an HTLV-1 antibody-containing plasma samples remained reactive up to a dilution of around 1:512,000 to 1:256,000, and an HTLV-2 antibody-containing plasma sample remained reactive to a dilution of 1:16,000. A plateau of high S/CO was observed in both across dilutions up to 1:16,000 and 1:4,096, respectively, which thereafter declined. When HTLV-1 OF specimens were similarly diluted, the limit of detection occurred at a dilution of 1:4,096, but the plateau was observed only to 1:64 for both samples. Regarding the HTLV-2 OF sample, the limit of detection was 1:8 and the plateau occurred up to 1:4. However, when HTLV-1 and HTLV-2 antibody-containing plasma samples were diluted in plasma pooled from seronegative donors, the S/CO declined rapidly and the samples became nonreactive at dilutions of 1:2,048 and 1:4, respectively, confirming that the reactivity is associated with the proportion of the target antibody to total antibody, rather than with their absolute concentration.
FIG 4.
Analytical sensitivity of the IgG capture assay based on 2-fold serial dilutions of anti-HTLV-1/2 antibody-containing plasma (A) and OF (B) in transport medium and that of antibody-containing plasma in plasma pooled from seronegative donors (C) until the limit of detection. The horizontal dashed line indicates the S/CO at 1.00, above which samples are considered reactive.
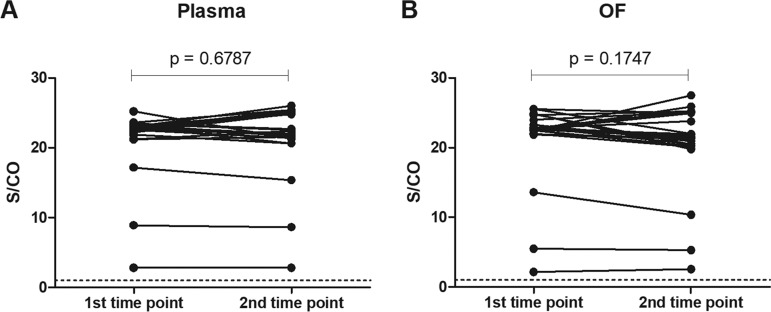
Paired samples collected at different time points from the same patients showed similar results for both plasma and OF using the IgG capture assay (P = 0.6787 and P = 0.1747) (Fig. 5).
FIG 5.
Variation of S/CO values over time in IgG capture assay. Samples obtained from 24 individuals, with an average of 3.3 months between the two time points. (A) plasma and (B) oral fluid. Horizontal dashed line indicates the S/CO at 1.00, above which samples are considered reactive. P value indicates degree of statistical significance.
Antibody reactivity levels by clinical phenotype.
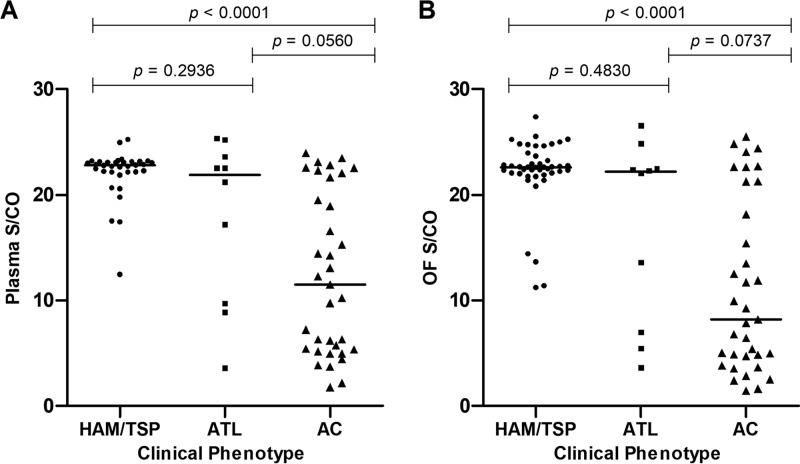
S/CO values of plasma and OF samples from infected patients with clinical phenotypes of HAM/TSP, ATL, or asymptomatic carriage (AC) of infection were compared (Fig. 6). Antibody reactivities were highest in specimens obtained from HAM/TSP patients (median S/CO for plasma, 22.6; for OF, 22.43) followed by those in ATL patients (median S/CO for plasma, 21.19; for OF, 22.18). Asymptomatic carriers had the lowest antibody reactivities (median S/CO for plasma, 11.49; for OF, 8.21), significantly lower than that of HAM/TSP patients (P < 0.0001).
FIG 6.
Comparison of S/CO values of plasma (A) and oral fluid (B) samples from HTLV-1-infected patients presenting with a clinical phenotype of HTLV-1-associated myelopathy (HAM/TSP) (n = 34), adult T cell leukemia/lymphoma (ATL) (n = 10), or asymptomatic carriage (AC) of infection (n = 35). Solid horizontal bars show median. P value indicates degree of statistical significance.
Stability of anti-HTLV IgG.
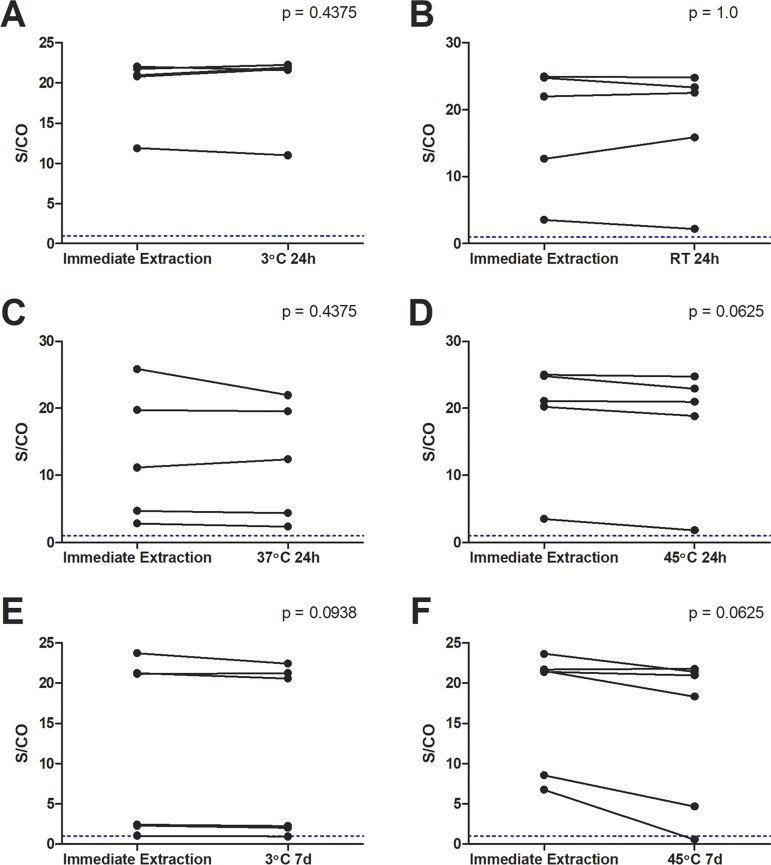
In preparation for field testing, OF samples were investigated for the stability of anti-HTLV-1/2 IgG capture reactivity under different simulated environmental conditions. Delaying processing of specimens (n = 5) at different temperatures for 24 h did not significantly affect their reactivity for anti-HTLV-1/2 IgG (3°C, P = 0.4375; room temperature, P = 1.00; 37°C, P = 0.4375; 45°C, P = 0.0625) (Fig. 7). When samples were held at 3°C and 45°C for 7 days (n = 6), there was no significant difference in reactivity (3°C, P = 0.0938; 45°C, P = 0.0625), although a single OF specimen at 45°C and one at 3°C became nonreactive from an S/CO of 6.79 and 1.05, respectively. Submitting processed OF samples to 5 or 10 consecutive freeze-thaw cycles also did not result in any loss in reactivity (S/CO freeze-thaw at 1×, 5×, 10×: sample with high S/CO = 24.8, 25.1; 27.4; sample with low S/CO: 5.3, 5.5, 5.7).
FIG 7.
Comparison of S/CO values between anti-HTLV-1/2 antibody-containing OF samples submitted to delayed processing at various temperatures (3°C, room temperature [RT], 37°C, and 45°C) for 24 h (24h) or 7 days (7d) and samples extracted on the day of collection. Each temperature condition was evaluated by five different paired OF specimens for 24 h and by 6 paired samples for 7 days. The horizontal dashed line indicates the S/CO at 1.00, above which samples are considered reactive. P value indicates degree of statistical significance.
DISCUSSION
This study evaluated the performance of an immunoglobulin (Ig) capture assay to detect anti-HTLV-1/2 IgG in GCF-rich OF using a commercially available horseradish peroxidase-labeled antigen. The assay was highly sensitive (100%) and specific (100%) on both OF and plasma and showed good reproducibility. For comparison, the commercially available Murex HTLV I+II EIA, which is optimized to detect anti-HTLV-1/2 antibodies in serum, was evaluated. This assay demonstrated 100% sensitivity using plasma, consistent with previous findings, but did not identify anti-HTLV-1/2 antibodies in 14% of OF specimens from infected patients (26). This observation is not surprising, since this double antigen-binding assay is configured solely for the analysis of serum or plasma.
The global burden of HTLV-1 infection is likely an underestimation, partially owing to a lack of population-based studies in many regions, including large parts of Asia and Africa (1). In particular, data concerning infections in children are limited (18). HTLV-1-infected children have a lifetime risk of developing ATL as high as 20% (27). In addition, they are at greater risk of developing infective dermatitis, juvenile HAM/TSP, juvenile ATL, and stunted physical growth (28–30). Indigenous populations of central Australia, Africa, and South America represent another challenge to conducting epidemiological studies, in part related to a reluctance in partaking in biomedical research (31). Among Indigenous Australians, HTLV-1-associated bronchiectasis contributes to poor clinical outcomes in adults, but little is known about pediatric prevalence (32). Moreover, the high asymptomatic viral carriage rate and prolonged clinical latency before manifestation of overt HTLV-1-associated disease further emphasizes the need for active surveillance of infection on a population basis.
Because OF contains serum-derived IgG, it has been evaluated as a noninvasive alternative to venous blood for serological diagnosis of various infections, including HIV, measles, mumps, rubella (MMR), and hepatitis A virus (33–35). OF collection is quick, straightforward, and can be conducted at home or in the community, allowing large numbers of samples to be collected in a short period of time. The use of OF for diagnosis has the additional advantages of not requiring the skills of a phlebotomist or of facilities to dispose of sharps, and it also provides, as we have shown, a relatively stable analyte for onwards transfer to a centralized testing facility. It has been shown to be well tolerated among children (15, 21). OF serology has the potential for high acceptance among Indigenous populations and to enable the large population-based studies of HTLV-1/2 infection needed to assess the impact of this infection. A pilot study should now be conducted to confirm the acceptability of OF collection in the field, especially among Indigenous populations.
Although OF contains considerably less IgG than serum and plasma, IgG capture assays detect even low concentrations of anti-HTLV-1/2 IgG in analytes under test. The advantage given by this format is that, as long as there is sufficient IgG to saturate the solid phase, the initial concentration is not important. Usually, for diagnostic purposes, it is necessary to validate samples as suitable for testing by demonstrating a minimum level of detectable IgG, but this is difficult to do in the field and is why initial studies should be undertaken to show that field sample pairing of OF and plasma results in similar S/CO reactivities. This was widely examined in recent studies using OF sampling to detect anti-Ebola virus antibody and showed a similar utility in field epidemiology without recourse to IgG measurement (36, 37).
The reactivity that a sample gives in an IgG capture assay depends not on absolute immunoglobulin titers per se but rather on the proportion of specific antibody in the IgG captured in the solid phase. Assays of this format display an extended plateau of reactivity, allowing further dilution in serum-free fluids and confirming a robustness to inadvertent dilution of the OF (Fig. 4). In contrast, in the Murex HTLV I+II double antigen-binding assay, sample reactivity is influenced by the absolute titer of the specific antibody (38) and when analytes other than serum/plasma are investigated. This feature of robustness of the IgG capture assay was highlighted by the strong positive correlation between paired OF and plasma S/CO reactivities, indicating that the assay detected similar proportions of anti-HTLV-1/2 IgG in both fluids, irrespective of differences in IgG levels, as previously described for anti-Ebola virus antibody (36). Thus, when anti-HTLV-1/2-containing plasma samples were diluted serially in a diluent not containing human IgG in the antibody capture assay, the S/CO remained unaffected across multiple dilutions, and anti-HTLV-1/2 IgG reactivity was detected up to at least a 1:256,000 dilution. A similar pattern was observed when OF from an HTLV-1/2-seropositive individual was diluted, although the S/CO declined from the saturation plateau and reached the limit of detection at lower dilutions. This is to be expected given the lower IgG content in OF, which results in the solid phase becoming desaturated earlier since the OF represents ab initio approximately a 1 in 500 to 1000 dilution of plasma. In contrast, anti-HTLV-1/2 antibody-containing plasma specimens diluted in plasma pooled from seronegative donors rapidly became nonreactive because of the presence of nonreactive IgG that reduced the proportion of anti-HTLV-1/2 IgG in the captured IgG. The greater diagnostic sensitivity of the IgG capture assay is a measure of the small amount of IgG required in the analyte to saturate the solid phase. The OF samples that were nonreactive in the Murex HTLV I+II EIA all had low levels of anti-HTLV-1/2 IgG.
As antibody capture assays represent the relative anti-HTLV-1/2 IgG proportions in the captured IgG, plasma S/CO values were used as a semiquantitative indication of antibody titer and compared between infected individuals of different clinical phenotypes. HAM/TSP patients exhibited the highest levels of anti-HTLV-1/2 IgG reactivity, in agreement with previous reports (39, 40). Interestingly, patients with ATL and asymptomatic carriers demonstrated greater heterogeneity of antibody reactivities. High levels of anti-HTLV-1/2 IgG in carriers have been identified as a risk factor for ATL (41). In contrast, low reactivity in some ATL patients may result from impaired humoral immunity secondary to either the disease or to antineoplastic therapy, as reported for acute lymphoblastic and acute myeloid leukemias (42, 43).
Another objective of this study was to determine the stability of OF anti-HTLV-1/2 IgG under various environmental conditions. HTLV-1/2 infections are commonly found in populations inhabiting tropical and subtropical regions, and samples could potentially be exposed to extremes of temperatures in transit. However, OF specimens submitted to temperatures between 3°C and 45°C for up to 7 days before processing did not show significant changes in anti-HTLV-1/2 IgG levels. Successive freeze-thawing of extracted OF also did not affect reactivities, consistent with previous findings that demonstrated stability of anti-MMR antibodies in serum after up to 10 freeze-thaw cycles (44). This is advantageous when, for example, samples need to be removed from storage for retesting or transportation. The stability of anti-HTLV-1/2 IgG reactivities could be because antibody capture assays remain sensitive to low immunoglobulin concentrations, such that even if antibody protein denaturation did occur, IgG measurements would not be significantly compromised, although this was not tested (45). It is important to highlight that optimal assay protocols should still consist of the immediate extraction of the oral fluid from foam swabs.
Clinical specimens evaluated in this study were obtained from individuals who might not be truly representative of those seen in areas where HTLV-1/2 are endemic, particularly in Africa, where coinfections with different pathogens are common. Indeed, HTLV-1 false positivity on serological assays, usually of the indirect immunoassay format, has been observed and postulated to arise through cross-reactive anti-Plasmodium falciparum antibodies (46). Moreover, the impact of oral health on the quality of IgG in OF has not been examined in depth, and HTLV-1 infection has been associated with poorer oral status characterized by reduced salivary flow, periodontitis, and gum-tooth detachment (47). Such pathologies are, however, likely to be associated with enhanced GCF IgG. Therefore, the OF and serum or plasma samples from individuals in regions where HTLV-1/2 are endemic should be evaluated to further validate the assay. In the current study, only one oral fluid sample from an HTLV-2-infected individual was included. The IgG capture assay correctly detected antibodies in this sample. All eight historical plasma samples from HTLV-2-infected patients were also reactive (data not shown). More oral fluid samples obtained from HTLV-2 patients should be included in the future to confirm the assay sensitivity for HTLV-2.
In summary, OF serological testing using the described HTLV-1/2 antibody capture EIA is highly sensitive and specific. OF represents a noninvasive alternative to venous blood for detecting anti-HTLV-1/2 IgG and opens the potential for large-scale community seroprevalence studies, even in remote areas.
ACKNOWLEDGMENTS
The contributions of all patients and volunteers who agreed to participate in this study and who generously consented to the collection of samples are gratefully acknowledged, along with the support of staff at the National Centre for Human Retrovirology who recruited the patients and obtained the samples. Public Health England provided anonymized OF samples for validation purposes.
G.P.T. is supported by the Imperial NIHR Biomedical Research Centre.
REFERENCES
- 1.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. 2005. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 3.Martin F, Taylor GP, Jacobson S. 2014. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol 10:1531–1546. doi: 10.1586/1744666X.2014.966690. [DOI] [PubMed] [Google Scholar]
- 4.Iwanaga M, Watanabe T, Yamaguchi K. 2012. Adult T-cell leukemia: a review of epidemiological evidence. Front Microbiol 3:322. doi: 10.3389/fmicb.2012.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beilke MA, Theall KP, O'Brien M, Clayton JL, Benjamin SM, Winsor EL, Kissinger PJ. 2004. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis 39:256–263. doi: 10.1086/422146. [DOI] [PubMed] [Google Scholar]
- 6.Gotuzzo E, Terashima A, Alvarez H, Tello R, Infante R, Watts DM, Freedman DO. 1999. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg 60:146–149. doi: 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 7.Blas M, Bravo F, Castillo W, Castillo WJ, Ballona R, Navarro P, Catacora J, Cairampoma R, Gotuzzo E. 2005. Norwegian scabies in Peru: the impact of human T cell lymphotropic virus type I infection. Am J Trop Med Hyg 72:855–857. doi: 10.4269/ajtmh.2005.72.855. [DOI] [PubMed] [Google Scholar]
- 8.Grassi MFR, dos Santos NP, Lírio M, Kritski AL, Chagas Almeida M. d C, Santana LP, Lázaro N, Dias J, Netto EM, Galvão-Castro B. 2016. Tuberculosis incidence in a cohort of individuals infected with human T-lymphotropic virus type 1 (HTLV-1) in Salvador, Brazil. BMC Infect Dis 16. doi: 10.1186/s12879-016-1428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araujo A, Hall WW. 2004. Human T-lymphotropic virus type II and neurological disease. Ann Neurol 56:10–19. doi: 10.1002/ana.20126. [DOI] [PubMed] [Google Scholar]
- 10.Murphy EL, Wang B, Sacher RA, Fridey J, Smith JW, Nass CC, Newman B, Ownby HE, Garratty G, Hutching ST, Schreiber GB. 2004. Respiratory and urinary tract infections, arthritis, and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis 10:109–116. doi: 10.3201/eid1001.020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimoyama M. 1991. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 12.De Castro-Costa CM, Araujo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, de Paula SM, Ishak R, Ribas JG, Rovirosa LC, Carton H, Gotuzzo E, Hall WW, Montano S, Murphy EL, Oger J, Remondegui C, Taylor GP. 2006. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses 22:931–935. doi: 10.1089/aid.2006.22.931. [DOI] [PubMed] [Google Scholar]
- 13.Jacob F, Santos-Fortuna E. d l, Azevedo RS, Caterino-de-Araujo A. 2007. Performances of HTLV serological tests in diagnosing HTLV infection in high-risk population of Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo 49:361–364. doi: 10.1590/s0036-46652007000600005. [DOI] [PubMed] [Google Scholar]
- 14.Kowal E, Greenwood A, McWhirter RE. 2015. All in the blood: a review of Aboriginal Australians’ cultural beliefs about blood and implications for biospecimen research. J Empir Res Hum Res Ethics 10:347–359. doi: 10.1177/1556264615604521. [DOI] [PubMed] [Google Scholar]
- 15.Vyse AJ, Cohen BJ, Ramsay ME. 2001. A comparison of oral fluid collection devices for use in the surveillance of virus diseases in children. Public Health 115:201–207. doi: 10.1038/sj.ph.1900751. [DOI] [PubMed] [Google Scholar]
- 16.Delaporte E, Dupont A, Peeters M, Josse R, Merlin M, Schrijvers D, Hamono B, Bedjabaga L, Cheringou H, Boyer F. 1988. Epidemiology of HTLV-I in Gabon (Western Equatorial Africa). Int J Cancer 42:687–689. doi: 10.1002/ijc.2910420509. [DOI] [PubMed] [Google Scholar]
- 17.Einsiedel L, Woodman RJ, Flynn M, Wilson K, Cassar O, Gessain A. 2016. Human T-lymphotropic virus type 1 infection in an Indigenous Australian population: epidemiological insights from a hospital-based cohort study. BMC Public Health 16:5. doi: 10.1186/s12889-016-3366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hlela C, Shepperd S, Khumalo NP, Taylor GP. 2009. The prevalence of human T-cell lymphotropic virus type 1 in the general population is unknown. AIDS Rev 11:205–214. [PubMed] [Google Scholar]
- 19.Mortimer PP, Parry JV. 1994. Detection of antibody to HIV in saliva: a brief review. Clin Diagn Virol 2:231–243. doi: 10.1016/0928-0197(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 20.Tamashiro H, Constantine NT. 1994. Serological diagnosis of HIV infection using oral fluid samples. Bull World Health Organ 72:135–143. [PMC free article] [PubMed] [Google Scholar]
- 21.Nokes DJ, Enquselassie F, Vyse A, Nigatu W, Cutts FT, Brown DW. 1998. An evaluation of oral-fluid collection devices for the determination of rubella antibody status in a rural Ethiopian community. Trans R Soc Trop Med Hyg 92:679–685. doi: 10.1016/S0035-9203(98)90811-2. [DOI] [PubMed] [Google Scholar]
- 22.Soto-Ramirez LE, Garcia-Vallejo F, Renjifo B, Vergara A, Borrero I, Marlink R, Essex M. 1995. Human T-lymphotropic virus type I (HTLV-I)-specific antibodies and cell-free RNA in crevicular fluid-rich saliva from patients with tropical spastic paraparesis/HTLV-I-associated myelopathy. Viral Immunol 8:141–150. doi: 10.1089/vim.1995.8.141. [DOI] [PubMed] [Google Scholar]
- 23.Belec L, Jean Georges A, Hallouin MC, Si Mohamed A, Morand-Joubert L, Georges-Courbot MC. 1996. Human T-lymphotropic virus type I excretion and specific antibody response in paired saliva and cervicovaginal secretions. AIDS Res Hum Retroviruses 12:157–167. doi: 10.1089/aid.1996.12.157. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez MC, Salcedo M, Garcia-Vallejo F. 2015. Serological and virological evaluation of human T-lymphotropic virus type 1 infection in family groups from Tumaco, Colombia. Biomedica 35:337–346. doi: 10.7705/biomedica.v35i3.2601. [DOI] [PubMed] [Google Scholar]
- 25.Achiron A, Higuchi I, Takenouchi N, Matsuoka E, Hashimoto K, Izumo S, Shohat B, Osame M. 1997. Detection of HTLV type I provirus by in situ polymerase chain reaction in mouthwash mononuclear cells of HAM/TSP patients and HTLV type I carriers. AIDS Res Hum Retroviruses 13:1067–1070. doi: 10.1089/aid.1997.13.1067. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Brito V, Santos FLN, Gonçalves NLS, Araujo THA, Nascimento DSV, Pereira FM, Boa-Sorte NCA, Grassi MFR, Caterino-de-Araujo A, Galvão-Castro B. 2018. Performance of commercially available serological screening tests for human T-cell lymphotropic virus infection in Brazil. J Clin Microbiol 56:18. doi: 10.1128/JCM.00961-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosadas C, Malik B, Taylor GP, Puccioni-Sohler M. 2018. Estimation of HTLV-1 vertical transmission cases in Brazil per annum. PLoS Negl Trop Dis 12:e0006913. doi: 10.1371/journal.pntd.0006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Grenade L, Manns A, Fletcher V, Derm D, Carberry C, Hanchard B, Maloney EM, Cranston B, Williams NP, Wilks R, Kang EC, Blattner WA. 1998. Clinical, pathologic, and immunologic features of human T-lymphotrophic virus type I-associated infective dermatitis in children. Arch Dermatol 134:439–444. doi: 10.1001/archderm.134.4.439. [DOI] [PubMed] [Google Scholar]
- 29.Bittencourt AL, Primo J, Oliveira MF. 2006. Manifestations of the human T-cell lymphotropic virus type I infection in childhood and adolescence. J Pediatr (Rio J) 82:411–420. doi: 10.2223/JPED.1573. [DOI] [PubMed] [Google Scholar]
- 30.Araújo APQC, Fontenelle LMC, Pádua PAB, Maia Filho HS, Araújo ADQC. 2002. Juvenile human T lymphotropic virus type 1-associated myelopathy. Clin Infect Dis 35:201–204. doi: 10.1086/341251. [DOI] [PubMed] [Google Scholar]
- 31.Glover M, Kira A, Johnston V, Walker N, Thomas D, Chang AB, Bullen C, Segan CJ, Brown N. 2015. A systematic review of barriers and facilitators to participation in randomized controlled trials by Indigenous people from New Zealand, Australia, Canada and the United States. Glob Health Promot 22:21–31. doi: 10.1177/1757975914528961. [DOI] [PubMed] [Google Scholar]
- 32.Einsiedel L, Fernandes L, Spelman T, Steinfort D, Gotuzzo E. 2012. Bronchiectasis is associated with human T-lymphotropic virus 1 infection in an Indigenous Australian population. Clin Infect Dis 54:43–50. doi: 10.1093/cid/cir766. [DOI] [PubMed] [Google Scholar]
- 33.Granade TC, Phillips SK, Parekh B, Gomez P, Kitson-Piggott W, Oleander H, Mahabir B, Charles W, Lee-Thomas S. 1998. Detection of antibodies to human immunodeficiency virus type 1 in oral fluids: a large-scale evaluation of immunoassay performance. Clin Diagn Lab Immunol 5:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DW, Ramsay ME, Richards AF, Miller E. 1994. Salivary diagnosis of measles: a study of notified cases in the United Kingdom, 1991–3. BMJ 308:1015–1017. doi: 10.1136/bmj.308.6935.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris-Cunnington MC, Edmunds WJ, Miller E, Brown DW. 2004. A population-based seroprevalence study of hepatitis A virus using oral fluid in England and Wales. Am J Epidemiol 159:786–794. doi: 10.1093/aje/kwh107. [DOI] [PubMed] [Google Scholar]
- 36.Tedder RS, Samuel D, Dicks S, Scott JT, Ijaz S, Smith CC, Adaken C, Cole C, Baker S, Edwards T, Kamara P, Kargbo O, Niazi S, Nwakanma D, d'Alessandro U, Burch G, Doughty H, Brown CS, Andrews N, Glynn JR, van Griensven J, Pollakis G, Paxton WA, Semple MG. 2018. Detection, characterization, and enrollment of donors of Ebola convalescent plasma in Sierra Leone. Transfusion 58:1289–1298. doi: 10.1111/trf.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glynn JR, Bower H, Johnson S, Houlihan CF, Montesano C, Scott JT, Semple MG, Bangura MS, Kamara AJ, Kamara O, Mansaray SH, Sesay D, Turay C, Dicks S, Wadoum REG, Colizzi V, Checchi F, Samuel D, Tedder RS. 2017. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect Dis 17:645–653. doi: 10.1016/S1473-3099(17)30111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parry JV. 1993. Simple and reliable salivary tests for HIV and hepatitis A and B virus diagnosis and surveillance. Ann N Y Acad Sci 694:216–233. doi: 10.1111/j.1749-6632.1993.tb18355.x. [DOI] [PubMed] [Google Scholar]
- 39.Burbelo PD, Meoli E, Leahy HP, Graham J, Yao K, Oh U, Janik JE, Mahieux R, Kashanchi F, Iadarola MJ, Jacobson S. 2008. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology 5:96. doi: 10.1186/1742-4690-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enose-Akahata Y, Abrams A, Johnson KR, Maloney EM, Jacobson S. 2012. Quantitative differences in HTLV-I antibody responses: classification and relative risk assessment for asymptomatic carriers and ATL and HAM/TSP patients from Jamaica. Blood 119:2829–2836. doi: 10.1182/blood-2011-11-390807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hisada M, Okayama A, Shioiri S, Spiegelman DL, Stuver SO, Mueller NE. 1998. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood 92:3557–3561. [PubMed] [Google Scholar]
- 42.Alanko S, Pelliniemi TT, Salmi TT. 1992. Recovery of blood B-lymphocytes and serum immunoglobulins after chemotherapy for childhood acute lymphoblastic leukemia. Cancer 69:1481–1486. doi:. [DOI] [PubMed] [Google Scholar]
- 43.Reilly A, Kersun LS, Luning Prak E, Boyer J, McDonald K, Jawad AF, Sullivan KE. 2013. Immunologic consequences of chemotherapy for acute myeloid leukemia. J Pediatr Hematol Oncol 35:46–53. doi: 10.1097/MPH.0b013e318266c0c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinsky NA, Huddleston JM, Jacobson RM, Wollan PC, Poland GA. 2003. Effect of multiple freeze-thaw cycles on detection of measles, mumps, and rubella virus antibodies. Clin Diagn Lab Immunol 10:19–21. doi: 10.1128/cdli.10.1.19-21.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris M, Cohen B, Andrews N, Brown D. 2002. Stability of total and rubella-specific IgG in oral fluid samples: the effect of time and temperature. J Immunol Methods 266:111–116. doi: 10.1016/s0022-1759(02)00114-x. [DOI] [PubMed] [Google Scholar]
- 46.Hayes CG, Burans JP, Oberst RB. 1991. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis 163:257–262. doi: 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- 47.Lins L, de Carvalho VJU, de Almeida Rego FF, Azevedo R, Kashima S, Gallazi VNO, Xavier MT, Galvão-Castro B, Alcantara LC Jr. 2012. Oral health profile in patients infected with HTLV-1: clinical findings, proviral load, and molecular analysis from HTLV-1 in saliva. J Med Virol 84:1428–1436. doi: 10.1002/jmv.23327. [DOI] [PubMed] [Google Scholar]